Abstract
A genetic approach was used to increase phosphatidylinositol(4,5)bisphosphate [PtdIns(4,5)P2] biosynthesis and test the hypothesis that PtdInsP kinase (PIPK) is flux limiting in the plant phosphoinositide (PI) pathway. Expressing human PIPKIα in tobacco (Nicotiana tabacum) cells increased plasma membrane PtdIns(4,5)P2 100-fold. In vivo studies revealed that the rate of 32Pi incorporation into whole-cell PtdIns(4,5)P2 increased >12-fold, and the ratio of [3H]PtdInsP2 to [3H]PtdInsP increased 6-fold, but PtdInsP levels did not decrease, indicating that PtdInsP biosynthesis was not limiting. Both [3H]inositol trisphosphate and [3H]inositol hexakisphosphate increased 3-and 1.5-fold, respectively, in the transgenic lines after 18 h of labeling. The inositol(1,4,5)trisphosphate [Ins(1,4,5)P3] binding assay showed that total cellular Ins(1,4,5)P3/g fresh weight was >40-fold higher in transgenic tobacco lines; however, even with this high steady state level of Ins(1,4,5)P3, the pathway was not saturated. Stimulating transgenic cells with hyperosmotic stress led to another 2-fold increase, suggesting that the transgenic cells were in a constant state of PI stimulation. Furthermore, expressing Hs PIPKIα increased sugar use and oxygen uptake. Our results demonstrate that PIPK is flux limiting and that this high rate of PI metabolism increased the energy demands in these cells.
INTRODUCTION
Inositol phospholipids are important for both sensing and responding to various stimuli in plants and animal cells. For example, phosphatidylinositol(4,5)bisphosphate [PtdIns(4,5)P2] is the source of the second messenger inositol(1,4,5)trisphosphate [Ins(1,4,5)P3], and it can directly affect the activity of membrane proteins, regulate cytoskeletal structure, and affect vesicle trafficking (for review, see Stevenson et al., 1998; Meijer and Munnik, 2003; Van Leeuwen et al., 2004). However, a long-standing mystery for plant scientists studying phosphoinositide (PI) signaling has been the low relative amount of PtdIns(4,5)P2 in all terrestrial plants studied thus far (Gross and Boss, 1993; Munnik et al., 1998; Stevenson et al., 1998; Drøbak et al., 1999).
The ratio of [3H]inositol-labeled PtdIns(4)P to PtdIns(4,5)P2 is usually on the order of 10:1, which is high compared with the 1:1 and 1:2 ratios characteristic of rapidly responding animal cells (Adel-Latif et al., 1985; Cunningham et al., 1995). The relatively low steady state level of PtdIns(4,5)P2 in plants could result from a low rate of phosphorylation of PtdIns(4)P by PtdIns(4)P 5-kinase to form PtdIns(4,5)P2 or from rapid hydrolysis by phospholipase C (PLC) or dephosphorylation by a phosphatase (Drøbak et al., 1999; Pical et al., 1999; Stevenson et al., 2000; Berdy et al., 2001; DeWald et al., 2001; Burnette et al., 2003; Hunt et al., 2003; Ercetin and Gillaspy, 2004; Zhong et al., 2004; Williams et al., 2005).
A clue as to the differences in plant and animal PI signaling came from studies of lipid kinase activities in membranes isolated from rats and plants (Sandelius and Sommarin, 1990). These early experiments revealed that rat plasma membranes had 20-fold higher specific activity relative to soybean (Glycine max) hypocotyl or wheat (Triticum aestivum) shoot plasma membranes. More recently, plant genes have been cloned and expressed as recombinant proteins for biochemical characterization (Mikami et al., 1998; Elge et al., 2001; Westergren et al., 2001; Perera et al., 2005). A kinetic analysis of two isoforms of Arabidopsis thaliana PtdInsP kinases (At PIPK1 and At PIPK10) indicated that the plant enzymes were significantly less active (Vmax/Km of 20- to 200-fold less, respectively) compared with the human enzyme, Hs PIPKIα (Perera et al., 2005).
A unique feature of plant type I PIPKs is the presence of several MORN motifs in the N-terminal region of the protein. The N-terminal MORN domain is critical for regulating PIPK activity, and it affects membrane attachment in vivo (Im et al., 2007). Furthermore, PtdInsP kinase activity is regulated by protein phosphorylation (Westergren et al., 2001). These results clearly indicate that PtdInsP2 synthesis in plants is under tight control.
It is also evident that maintaining normal/adequate PtdInsP2 levels is important for different aspects of plant growth and development. For example, a continuous supply of PtdIns(4,5)P2 mediated through PtdIns 4-kinase or lipid transfer proteins is essential for normal root hair growth (Vincent et al., 2005; Preuss et al., 2006). Furthermore, the PI phosphatase sac9 mutant had 4-fold higher PtdInsP2 levels in root tissue and decreased primary and lateral root growth (Williams et al., 2005). An Arabidopsis PIPK mutant pip5k9-d (which has a mutation in the 3′ untranslated region of the gene, resulting in increased transcript levels) showed reduced primary root growth (Lou et al., 2007); however, it was not reported whether these changes were a result of changes in PtdInsP2 levels. Finally, tip growth in pollen tubes is also dependant on PtdInsP2 turnover (Dowd et al., 2006; Helling et al., 2006). These data suggest that the PI pathway is important for membrane trafficking and plasma membrane biogenesis.
In previous work, we found that plant cells transformed with the human type I InsP 5-phosphatase [which increased the rate of turnover of Ins(1,4,5)P3] showed increased endogenous PIPK activity. However, PLC activity was not altered (Perera et al., 2002). These results led to the hypothesis that the phosphorylation of PtdIns(4)P by PtdIns(4)P 5-kinase is a flux-limiting step in the plant PI pathway. To test this hypothesis, we have taken a synthetic biology approach. Using a well-characterized model plant system with relatively low PtdIns(4,5)P2, tobacco (Nicotiana tabacum) cells grown in suspension culture (Perera et al., 2002), we expressed a human PtdIns(4)P 5-kinase gene, Hs PIPKIα. We chose Hs PIPKIα because At PIPK1 and 10, the plant PIPKs characterized thus far, are much less active than Hs PIPKIα (Perera et al., 2005) and because Hs PIPKIα is a type I family PIPK (Kunz et al., 2000, 2002), which is functionally similar to the plant PIPKs (Mueller-Roeber and Pical, 2002). Importantly, Hs PIPKIα lacks MORN motifs; therefore, expressing Hs PIPKIα would increase PIPK activity without affecting MORN motif-interacting partners of At PIPK1-9. For comparison to the human enzyme, we have expressed At PIPK10, which does not contain MORN motifs.
In this article, we show that expressing Hs PIPKIα increases the plasma membrane PtdIns(4,5)P2 100-fold and generates a steady state increase in Ins(1,4,5)P3 that is similar to what is experienced transiently upon stimulation in nontransformed cells. These insights reveal the impact of increased PtdInsP2 biosynthesis on cellular metabolism. Because the transgenic cells are in a constant state of PI stimulation, they provide a good model for studying PI signaling.
RESULTS
Expression of PIPKs in Tobacco Cells
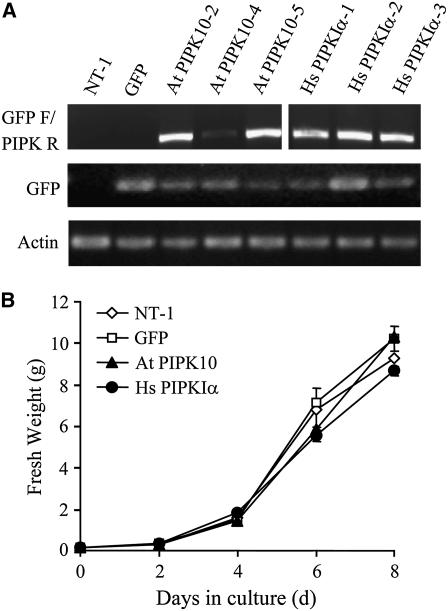
Vector constructs with either green fluorescent protein (GFP)-Hs PIPKIα, GFP-At PIPK10, or GFP alone were used for Agrobacterium tumefaciens–mediated transformation of the wild-type tobacco (NT-1) cells as previously described (Perera et al., 2002). Three independent lines producing GFP-Hs PIPKIα and GFP-At PIPK10 (hereafter referred to as Hs PIPKIα and At PIPK10) along with a GFP control line were selected for further study. Transcript levels were determined using gene-specific primers for GFP and the PIPKs (Figure 1A).
Figure 1.
Expression of GFP-PtdInsP 5-Kinase Genes in the Transgenic Tobacco Cells.
(A) RT-PCR analysis. RNA was isolated from wild-type and transgenic tobacco cell lines harvested on day 4 of the culture cycle. Equal amounts of RNA from each sample were reverse transcribed and subjected to RT-PCR using GFP, PIPK, and actin-specific primers. The full-length fusion transcripts were identified with a combination of GFP forward and the selective kinase reverse primers (top panel). Amplification of actin is shown to indicate that equal amounts of RNA were used from each sample. NT-1 (wild type); GFP (GFP vector control); At PIPK10-2, At PIPK10-4, and At PIPK10-5 (three independent transgenic lines transformed with GFP fused to Arabidopsis PIPK10); Hs PIPKIα-1, Hs PIPKIα-2, and Hs PIPKIα-3 (three independent transgenic lines transformed with GFP fused to human PIPKIα).
(B) Growth curve of wild-type and transgenic cell lines over the culture cycle. The fresh weight of two replicate 25-mL cultures was measured on each day. The values are averages (sd) of duplicates from two experiments.
Cell growth was compared over the normal 7-d growth cycle (Figure 1B). For all subsequent experiments, cells were grown under similar conditions, and the fresh weight of wild-type and transgenic cell lines did not vary more than 10%.
Subcellular Distribution of the Recombinant GFP-PIPKs
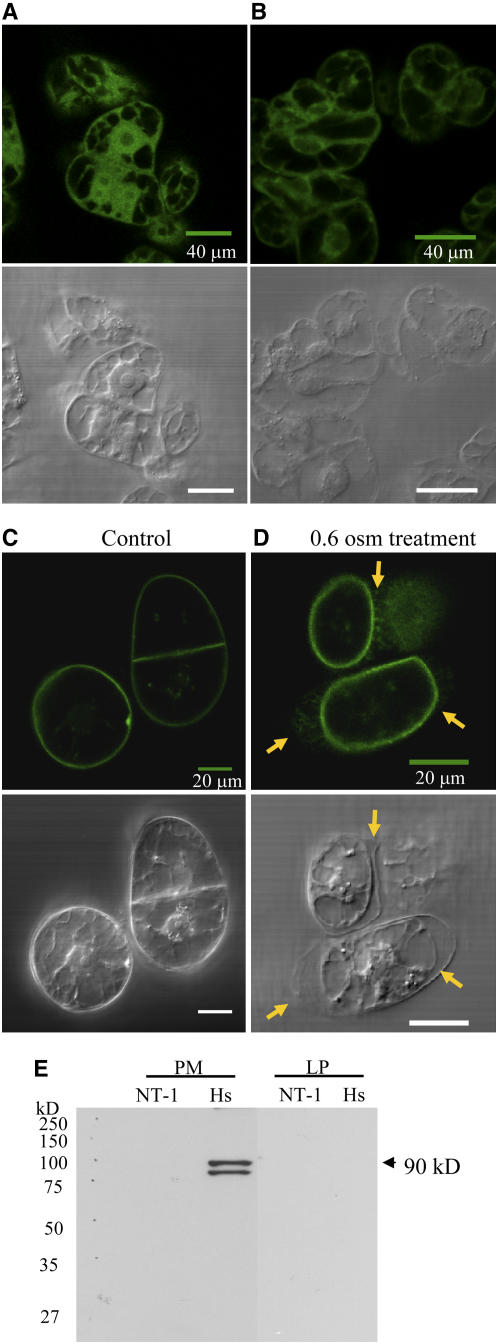
To determine the subcellular distribution of the GFP-PIPK proteins, the cells were imaged using a confocal microscope. Fluorescence was visible throughout the cytosol and nucleus for the GFP-expressing tobacco cells (Figure 2A) as previously described (Haseloff and Amos, 1995; Persson et al., 2002). By contrast, At PIPK10 was detected throughout the cytoplasmic strands but not in the nucleus (Figure 2B), and Hs PIPKIα was associated with the plasma membrane of the tobacco cells (Figure 2C; see Supplemental Video 1 online). To confirm plasma membrane localization of Hs PIPKIα, tobacco cells were treated with 0.6 osmolal sorbitol for 5 min (Figure 2D). Note the fluorescence remains with the plasma membrane and can be seen on the Hechtian threads, plasma membrane extensions that remain tightly associated with the cell wall (indicated by the arrows in Figure 2D and in Supplemental Video 2 online).
Figure 2.
Full-Length GFP-Hs PIPKIα Localized with the Plasma Membrane.
(A) to (C) Transgenic tobacco cells expressing GFP (A) and GFP-At PIPK10 (B), and GFP-Hs PIPKIα (C) were imaged using a confocal microscope. Top panels show fluorescence, and bottom panels show differential interference contrast images.
(D) To confirm plasma membrane localization of Hs PIPKIα, tobacco cells were treated with 0.6 osmolal sorbitol for 5 min. Arrows indicate the position of the cell wall.
(E) Immunoblot of plasma membrane (PM) and lower-phase (LP) proteins using a monoclonal antibody raised against GFP. The antiserum recognizes a protein of ∼90 kD (the predicted molecular weight of GFP-Hs PIPKIα) and a lower band that may be a proteolytic product in the plasma membrane of Hs PIPKIα cells. Equal amounts of membrane proteins were loaded from NT-1 (wild-type tobacco cells) and Hs (GFP-Hs PIPKIα lines).
Immunoblot analysis of proteins isolated from 4-d-old tobacco cells visualized with antiserum recognizing the GFP tag is shown in Figure 2E. Membranes were isolated by aqueous two-phase partitioning and proteins separated by gel electrophoresis. Hs PIPKIα was detected at the predicted size of the protein (∼90 kD) in the plasma membrane fraction and not in the lower phase membrane fraction by aqueous two-phase partitioning. These data confirmed that Hs PIPKIα was localized to the plasma membrane. Unlike Hs PIPKIα, At PIPK10 could not be detected by immunoblotting from a soluble, plasma membrane or lower-phase membrane fraction probably due to the low abundance of expressed protein (data not shown).
Expressing the PIPKs Increased PtdInsP 5-Kinase Activity and PtdIns(4,5)P2
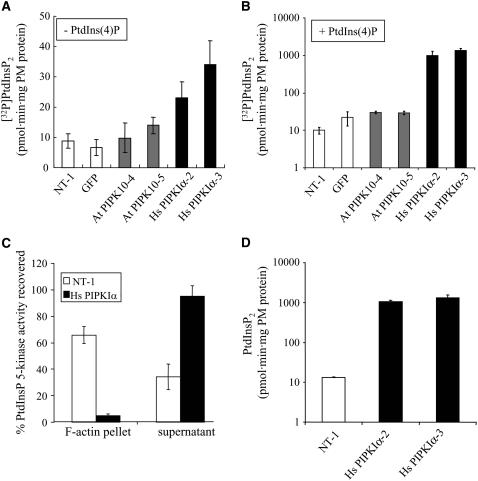
To determine whether expression of the At PIPK10 or Hs PIPKIα increased PtdInsP 5-kinase activity in tobacco cells, we first measured the plasma membrane lipid kinase activity in vitro. Two- to 3-fold more [32P]PtdIns(4,5)P2 was formed by membranes from two independent Hs PIPKIα lines even without adding exogenous PtdIns(4)P (Figure 3A). These data indicate that PtdIns(4)P was not limiting in the transgenic lines and that the human enzyme could phosphorylate the endogenous plant lipids. When PtdIns(4)P was added in excess, the specific activity of the isolated plasma membranes was 100-fold greater in the Hs PIPKIα lines and 1.5- to 2-fold greater in the At PIPK10 lines compared with the untransformed NT-1 plasma membranes (Figure 3B).
Figure 3.
Plasma Membrane PtdInsP 5-Kinase–Specific Activity and PtdIns(4,5)P2 Levels Increased >100-Fold in Hs PIPKIα Lines and Was Not Associated with Actin.
(A) and (B) The plasma membrane (PM)–enriched fraction from wild-type and two different lines of transgenic tobacco cells were analyzed for PtdInsP 5-kinase activity without (A) and with (B) added substrate, PtdIns(4)P.
(C) The PtdInsP 5-kinase activity recovered with F-actin polymerized and recovered in a 20,000g pellet from the plasma membrane of NT-1 and Hs PIPKIα lines was compared.
(D) Mass measurements of plasma membrane PtdIns(4,5)P2 levels of wild-type and Hs PIPKIα lines. The values plotted are the averages ± sd of two to three independent experiments assayed in duplicate. Note the data in (B) and (D) are plotted on a log scale.
Plant plasma membrane PtdInsP 5-kinase activity was reported previously to cosediment with the F-actin pellet isolated from carrot (Daucus carota) plasma membranes (Tan and Boss, 1992), and At PIPK1 has been shown to bind actin directly (Davis et al., 2007). With the wild-type tobacco cells, 66% ± 6% of the total plasma membrane PtdInsP 5-kinase activity cosedimented with the 20,000g F-actin pellet. However, of the plasma membrane PtdInsP 5-kinase activity from the Hs PIPKIα lines, 95% ± 8% was recovered in the detergent-soluble fraction and only 5% ± 1% sedimented with 20,000g F-actin pellet (Figure 3C). The fact that Hs PIPKIα did not cosediment with large F-actin bundles results from both the lack of the appropriate actin binding proteins necessary to form an Hs PIPKIα-actin scaffold and the inability of Hs PIPKIα to directly bind actin (Davis et al., 2007). The data confirm that Hs PIPKIα is associated with the plasma membrane and suggest that overproducing Hs PIPKIα should result in increased PtdIns(4,5)P2 in the plasma membrane, unless catabolism by PLC or a lipid phosphatase is greater than the rate of synthesis.
To monitor endogenous levels of PtdIns(4,5)P2 in the plasma membrane of transgenic tobacco cells in vivo, we used mass measurement. For these analyses, lipids were extracted from isolated plasma membranes from wild-type and Hs PIPKIα lines, the head group was hydrolyzed, and the total Ins(1,4,5)P3 released was monitored using the Ins(1,4,5)P3 binding assay. The plasma membrane PtdIns(4,5)P2 from the Hs PIPKIα lines increased >100-fold per mg membrane protein, from ∼10 to 1000 pmol·mg protein (Figure 3D).
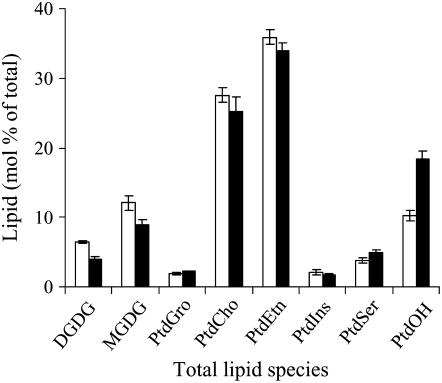
We also monitored steady state levels of the major phospholipids in the plasma membrane (Figure 4). Plasma membrane–enriched fractions were isolated from 4-d-old wild-type and transgenic tobacco cells and subjected to lipid extraction and profiling as described by Welti et al. (2002). The major structural lipids of the membrane, such as PtdGro, PtdEtn, PtdIns, PtdCho, and PtdSer, were not significantly different between wild-type and Hs PIPKIα cells. The most significant difference was the ∼1.8-fold increase in PtdOH in the Hs PIPKIα cells. In addition to the phospholipids, the plasma membrane digalactosyldiacylglycerol and monogalactosyldiacylglycerol levels were decreased by ∼30% in the Hs PIPKIα cells compared with the wild type.
Figure 4.
Polar Lipid Classes (Mol % of Total Polar Glycerolipids Analyzed) in the Plasma Membrane of Wild-Type and Hs PIPKIα Lines.
The major phospholipid classes (phosphatidylcholine [PtdCho], phosphatidylethanolamine [PtdEtn], phosphatidylglycerol [PtdGro], and phosphatidylinositol [PtdIns]), galactolipid classes (monogalactosyldiacylglycerol [MGDG] and digalactosyldiacylglycerol [DGDG]), and minor phospholipid classes (phosphatidylserine [PtdSer] and phosphatidic acid [PtdOH]) were present. Values are average ± se of duplicates from three independent experiments. Open bars, wild-type lines; closed bars, Hs PIPKIα lines.
Increased Turnover of PI Pathway Intermediates and Increased Rate of PtdInsP2 Synthesis in Vivo
To monitor the levels of intermediates in the PI pathway, tobacco cells were labeled in vivo with [3H]inositol (Table 1). Even though 20% of [3H]inositol can be incorporated into pectin and other cell wall polysaccharides (Verma and Maclachlan, 1976), in vivo labeling will give a relative measure of pool sizes for the major components of the PI pathway. The ratio of PtdIns(4)P to PtdIns(4,5)P2 in wild-type tobacco cells was ≥10:1. Expression of Hs PIPKIα increased the total cellular [3H]PtdIns(4,5)P2, and the ratio of [3H]PtdIns(4)P to [3H]PtdIns(4,5)P2 decreased to 2:1 without decreasing the total [3H]PtdIns(4)P. Analysis of the glycerophosphoinositol head groups confirmed the presence PtdIns4P and PtdIns(4,5)P2 (see Supplemental Figure 1 online). These data indicate that the PtdIns(4)P pool can be maintained at an adequate level to meet the demands of the increased PtdIns(4,5)P2 biosynthesis. It also supports the hypothesis that under normal conditions, PIPK activity is a flux-limiting step in the PI pathway of the wild-type cells.
Table 1.
Analysis of the [3H] Inositol Lipids from Wild-Type (NT-1) and Transgenic Tobacco Cells Expressing PIPKs
| [3H] Inositol–Labeled Phospholipids
|
||||
|---|---|---|---|---|
| (Percentage of Total [3H] Lipid Recovered)
|
Ratio
|
|||
| Cell Type | PtdIns | PtdInsP | PtdInsP2 | PtdInsP/PtdInsP2 |
| NT-1 | 91 ± 0.9 | 2.7 ± 0.9 | 0.24 ± 0.09 | 12:1 |
| GFP | 89 ± 1.2 | 3.0 ± 0.7 | 0.20 ± 0.07 | 15:1 |
| At PIPK10 | 88 ± 1.2 | 3.2 ± 0.5 | 0.27 ± 0.06 | 12:1 |
| Hs PIPKIα | 87 ± 1.5 | 2.4 ± 0.5 | 1.70 ± 0.90 | 2:1 |
Tobacco cells were labeled in vivo with myo-[2-3H]inositol for 24 h, and the lipids were extracted and separated by TLC. The values for [3H] labeled PtdIns, PtdInsP, and PtdInsP2 are the percentage of total radioactivity recovered (averages ± sd of two independent experiments assayed in duplicate).
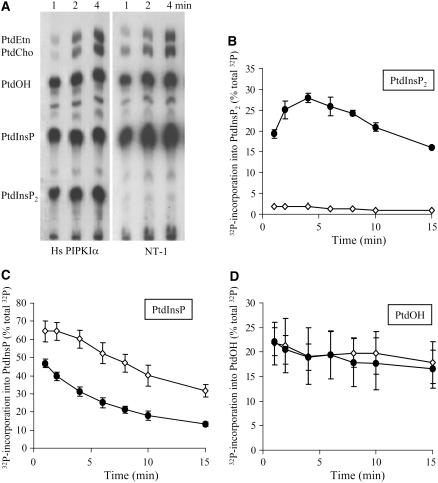
The rate of [32P]PtdIns(4,5)P2 biosynthesis in vivo was measured by adding 32Pi to 4-d-old transgenic Hs PIPKIα and wild-type tobacco cells preequilibrated in conditioned medium. Cells were harvested at each time point, and whole-cell lipids were extracted and separated by thin layer chromatography (TLC) (Figure 5A). Typically, little [32P]PtdIns(4,5)P2 was detected in the nontransformed cells. The incorporation of 32Pi into PtdIns(4,5)P2 in Hs PIPKIα lines was 12-fold higher than wild-type cells (Figure 5B) and saturated by 4 min when calculated as total [32P]-labeled lipids. The incorporation of 32Pi into PtdInsP was ∼20% less in Hs PIPKIα lines when calculated as percentage total [32P]-labeled lipids (Figure 5C). This reflects the high percentage of [32P]PtdIns(4,5)P2 and the rapid conversion of [32P]PtdInsP to [32P]PtdIns(4,5)P2. Comparison of these data with long-term [3H] labeling indicates that de novo synthesis of [3H]PtdInsP from intracellular pools was adequate to sustain [3H] PtdIns(4,5)P2 biosynthesis.
Figure 5.
In Vivo Labeling with 32Pi Indicates a Rapid Rate of [32P]PtdInsP2 Biosynthesis in the Hs PIPK1α Lines.
All cells were preequilibrated in conditioned medium for 30 min; 32Pi was added, cells were harvested, and lipids were extracted at the time points indicated. The lipids were separated by TLC, and 32P-labeled lipids were quantified with a Bioscan imaging scanner.
(A) Representative autoradiogram of the TLC plate.
(B) to (D) 32P recovered phospholipids (PtdInsP2, PtdInsP, and PtdOH, respectively) over the time course (wild type, open diamonds; Hs PIPKIα lines, closed circles). The data are reported as percentage of total cpm recovered per lane. Each point is the average ± sd of duplicates from three independent experiments.
As expected, in both cell lines, the [32P]PtdInsP decreased with time as the total percentage of [32P]-labeled phospholipids because of the increased incorporation of [32P] into PtdCho and PtdEtn. The observation that similar amounts of whole-cell [32P]PtdOH formed in all lines (Figure 5D) is a good indication that the cells were not stressed during the labeling (Welti et al., 2002) and that the increases in 32Pi incorporation were specific for PtdIns(4,5)P2.
Expressing the PIPKs Increased the Steady State Ins(1,4,5)P3
To determine whether increasing the rate of synthesis and the total mass of PtdIns(4,5)P2 in the plasma membranes would affect the total cellular Ins(1,4,5)P3, Ins(1,4,5)P3 levels were measured in wild-type and PIPK transgenic lines using the Ins(1,4,5)P3 binding assay (Table 2). In the Hs PIPKIα lines, the cellular Ins(1,4,5)P3 was >40-fold higher than that of the wild-type lines, and in the At PIPK10 lines, the total cellular Ins(1,4,5)P3 was 1.5- to 2-fold higher based on the Ins(1,4,5)P3 binding assay.
Table 2.
Basal Ins(1,4,5)P3 Was >40-Fold Higher in the Hs PIPKIα Cells and Increased Further in Response to Osmotic Stress
| Cell Type | Control (pmol InsP3/g FW) | Osmotic Stress (pmol InsP3/g FW) | Fold Increase in InsP3 |
|---|---|---|---|
| NT-1 | 139 ± 57 | 3,099 ± 366 | 25.5 ± 11.0 |
| GFP | 138 ± 47 | 4,336 ± 327 | 34.6 ± 6.0 |
| At PIPK10-4 | 328 ± 125 | 3,252 ± 1,145 | 10.1 ± 0.5 |
| At PIPK10-5 | 239 ± 4 | 2,442 ± 229 | 10.2 ± 3.0 |
| Hs PIPKIα-2 | 6,457 ± 1,692 | 16,679 ± 4,598 | 2.6 ± 0.1 |
| Hs PIPKIα-3 | 5,260 ± 106 | 13,427 ± 4,893 | 3.7 ± 1.4 |
Cells (0.25 g fresh weight [FW]) were preequilibrated in 5 mL of conditioned medium and harvested at time zero (control) and 15 min after adding 0.6 osmolal sorbitol. Ins(1,4,5)P3 was quantified using the Ins(1,4,5)P3 binding assay. Data are the averages ± sd from three separate experiments.
When the cells were stimulated by a hyperosmotic stress of 0.6 osmolal sorbitol, the total Ins(1,4,5)P3 was higher in all cell lines (Table 2). Ins(1,4,5)P3 levels in the unstimulated Hs PIPKIα lines were in a similar range to those of the stimulated nontransformed lines. In spite of these high levels, the Ins(1,4,5)P3 increased >2.5-fold when the Hs PIPKIα lines were stimulated. These data indicated that the capacity to hydrolyze PtdIns(4,5)P2 by PLC was not saturated in the Hs PIPKIα lines and indicate that the cells are in a continuously stimulated state with regard to PI signaling.
To compare the relative levels of [3H]inositol phosphates, cells were labeled with myo-[2-3H]inositol for 18 h, and water-soluble inositol phosphates were extracted and separated by HPLC. In addition to the increase in [3H]InsP3, [3H]InsP6 also was significantly higher in the Hs PIPKIα lines (Table 3).
Table 3.
Hs PIPKIα Lines Produced More [3H]InsPxs
| [3H] Inositol Phosphates (Percentage of Total [3H]InsPx Recovered from Water-Soluble Fraction)
|
||||
|---|---|---|---|---|
| Cell Type | InsP2 | InsP3 | InsP5 | InsP6 |
| NT-1 | 8.1 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.3 | 6.4 ± 0.2 |
| GFP | 7.3 ± 0.3 | 0.7 ± 0.2 | 1.2 ± 0.4 | 6.6 ± 0.3 |
| Hs PIPKIα | 6.7 ± 0.7 | 2.0 ± 0.3 | 1.1 ± 0.7 | 9.4 ± 1.0 |
To analyze the inositol phosphates from wild-type (NT-1) and transgenic cell lines, tobacco cells were labeled in vivo with myo-[2-3H]inositol for 18 h, and the water-soluble inositol phosphates were separated by HPLC and analyzed. [3H]InsP4 was not detected.
Cells with Increased PI Turnover Require More Calcium to Grow
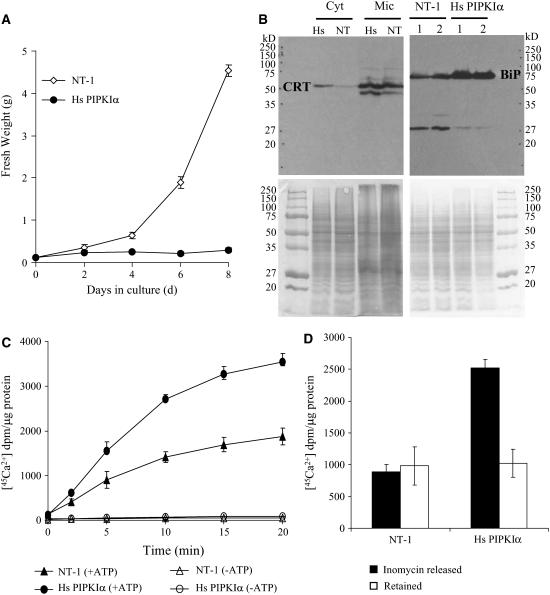
The increased flux through the PI pathway and constantly high Ins(1,4,5)P3 in the Hs PIPKIα lines should mobilize and may deplete the intracellular calcium stores. If the calcium stores were depleted, it might be expected that the Hs PIPKIα cells would grow less in medium with low calcium since calcium is essential for growth (Persson et al., 2001; Wu et al., 2002; Cheng et al., 2005). Cell growth was compared over the growth cycle in culture medium lacking calcium. While wild-type NT-1 cells grow less without added calcium in the medium (compared with normal medium, Figure 1B, which contains 3 mM Ca2+), the Hs PIPKIα cells did not grow at all without added Ca2+ (Figure 6A). These data suggested that the Hs PIPKIα cells lacked the calcium reserves of the wild-type cells.
Figure 6.
Hs PIPKIα Lines Did Not Grow in Medium with No Added Calcium and Showed Signs of Changes in Calcium Homeostasis.
(A) Cells (0.25 g/fresh weight) were transferred to 25 mL of fresh medium without added calcium, and fresh weight was monitored every 2 d (wild type, open diamonds; Hs PIPKIα lines, closed circles). The values are averages ± sd of duplicates from two experiments.
(B) Hs PIPKIα cells have increased CRT and BiP. Microsomal membranes (Mic) and cytosolic (Cyt) proteins were prepared from indicated cell lines, and 10 and 30 μg of protein were separated by SDS-PAGE as noted. The calcium binding proteins CRT and BiP were detected with antibodies by immunoblotting as indicated.
(C) Increased Ca2+ uptake in ER from Hs PIPK Iα cells. ER-enriched membrane vesicles were prepared from the wild type (triangles) and Hs PIPK Iα (circles) as previously described (Persson et al., 2001). [45Ca2+] (2 μCi) was added, and uptake was monitored. The ATP-dependent [45Ca2+] uptake (10 μg protein aliquot−1) was measured in the presence (closed symbols) and absence (open symbols) of 3 mM ATP. The radioactivity was measured in a scintillation counter. Data are averages ± sd of duplicates from two independent experiments.
(D) Hs PIPK Iα cells released more ER [45Ca2+]. The Ca2+ ionophore ionomycin (1.5 μM) was added to [45Ca2+]-loaded ER-enriched membrane vesicles (23 min), and membrane vesicles were analyzed for [45Ca2+] after 5 min. Closed bars, [45Ca2+] released; open bars, [45Ca2+] retained by the ER-enriched membranes. The values are averages ± sd of duplicates from two experiments.
The endoplasmic reticulum (ER) chaperones and calcium binding proteins, calreticulin (CRT) and binding protein (BiP), will bind endogenous calcium (Lievremont et al., 1997; Michalak et al., 1999). There was a significant increase in both CRT and BiP in the Hs PIPKIα lines in cells grown in normal medium with 3 mM calcium (Figure 6B). CRT increases with ER stress and when overproduced will increase ER-Ca2+ stores (Michalak et al., 1999; Persson et al., 2001; Akesson et al., 2005). The fact that the Hs PIPKIα lines did not grow on low Ca2+ medium suggests that they had low not high Ca2+ reserves (Wu et al., 2002; Cheng et al., 2005) and that the increase in CRT reflects an effort by the cells to bind and retain the remaining Ca2+ against the constant stimulus.
In vitro assays indicated that both ER and mitochondrial fractions from the Hs PIPKIα cells showed a 2-fold increase in [45Ca2+] uptake compared with the wild-type cells (Figure 6C; see Supplemental Figure 2A online). The enhanced uptake in the in vitro assays is consistent with the depletion of endogenous stored Ca2+ (i.e., increased number of available Ca2+ binding sites). After 20 min when [45Ca2+] uptake reached equilibrium, the Ca2+/H+ ionophore, ionomycin, was added to the membranes to release the ER-accumulated Ca2+ pool. As seen in Figure 6D, the ionomycin-released Ca2+ was ∼2.5 to 3-fold higher in Hs PIPKIα cells compared with the wild type. This increase in ionomycin-releasable Ca2+ is similar to that observed when CRT was overexpressed in NT-1 cells and is consistent with Ca2+ binding to the low-affinity binding sites on CRT (Persson et al., 2001).
An increase in Ca2+ flux also was detected in in vivo labeling studies using whole cells. If exposed to [45Ca2+], the Hs PIPKIα cells took up slightly more [45Ca2+] than wild-type cells (see Supplemental Figure 2B online). The growth study and [45Ca2+] data indicate that while the Hs PIPKIα cells have increased their capacity to store calcium, because of the constitutively high levels of InsP3, there will be a net efflux of Ca2+ and the cells require more exogenous calcium to survive.
We were unable to detect significant differences in cytosolic free calcium in whole cells using Indo-1 to monitor free calcium (see Supplemental Figure 3 online). When we measured total cellular Ca2+ using inductively coupled plasma (ICP) emission spectrometry, the Hs PIPKIα cells had 15.7% ± 1.1% and 15.6% ± 6.8% less Ca2+ per g dry weight at 4 and 6 d, respectively, compared with the NT-1 cells (values are the average of four numbers from two experiments). The combination of in vitro and in vivo measurements makes a compelling argument that the increase in PI metabolism in the Hs PIPKIα cells has increased the flux of Ca2+, resulting in a net loss of cellular Ca2+ and depletion of intracellular stores.
Increasing the Flux through the PI Pathway Increases Basal Metabolism
PtdIns(4,5)P2 biosynthesis from PtdIns requires two molecules of ATP; therefore, rapid turnover of the PI pathway should increase the demand for ATP (Poggioli et al., 1983; Yeung et al., 2006). In addition, InsP6 may be synthesized in plants by multiple routes that require ATP (Brearley and Hanke, 1996; Phillippy, 1998; Raboy, 2001; Stevenson-Paulik et al., 2002). Our in vivo labeling data provide support for the sequential phosphorylation of Ins(1,4,5)P3 to Ins(1,2,3,4,5,6)P6. This increased demand for ATP and an increased demand for energy as a result of increased Ca2+ flux should increase respiration in the transgenic tobacco cells. To monitor respiration in vivo, we used a Clarke oxygen electrode and measured oxygen uptake over time. As shown in Table 4, oxygen uptake by whole cells increased 40% in the Hs PIPKIα line. Although the trend was consistently higher, the increase in oxygen uptake in the At PIPK10 line was not statistically significant when averaged between experiments. With all cell lines, O2 uptake was completely inhibited by iodoacetate, a general inhibitor that also inhibits glycolysis (data not shown). The mitochondria of the wild-type and transgenic cells were visualized with Rhodamine 123 (see Supplemental Figure 4 online). There was no marked increase in the number of mitochondria and no difference in O2 uptake of isolated mitochondria (data not shown). Based on these observations, we hypothesized that the rate of glycolysis increased in the Hs PIPKIα line due to the increased PtdIns(4,5)P2 biosynthesis. If this were true, then the Hs PIPKIα line should use up the sugar from the culture medium faster. Analysis of the sugar in the culture medium supported this hypothesis. At day 6, there was half as much sugar remaining in the medium of the Hs PIPKIα lines compared with wild-type and GFP control lines (Table 5).
Table 4.
Cellular Respiration Increases in the Hs PIPKIα Lines
| Cell Type | nmoles O2 Taken up/min | Increased O2 Uptake (%) |
|---|---|---|
| NT-1 | 8 ± 3 | – |
| GFP | 8 ± 3 | – |
| At PIPK10 | 11 ± 2 | 26 |
| Hs PIPKIα | 13 ± 3 | 46 |
O2 uptake was monitored using a Clarke electrode in fresh medium. The numbers are the averages ± sd from three independent experiments.
Table 5.
Hs PIPKIα Lines Deplete the Sugar from the Medium Faster
| μg of Sugar/mL Conditioned Media
|
||
|---|---|---|
| Cell Type | 4 d | 6 d |
| NT-1 | 18.2 ± 0.1 | 6.2 ± 0.1 |
| GFP | 17.9 ± 0.4 | 6.0 ± 0.1 |
| At PIPK10 | 16.8 ± 1.2 | 6.9 ± 0.1 |
| Hs PIPKIα | 17.1 ± 0.1 | 3.4 ± 0.1 |
Sugar remaining in the culture medium was measured at the indicated day after transfer using Anthrone reagent as described in Methods. Data are the averages ± sd of duplicate values from two independent experiments.
DISCUSSION
Our first goal was to test the hypothesis that phosphorylation of PtdIns(4)P to form PtdIns(4,5)P2 by a PtdIns(4)P 5-kinase is a limiting step in plant PI metabolism. We showed that even though PtdIns(4)P is present and can be phosphorylated, the endogenous plant PIPKs are not as effective as the animal enzyme and that these differences are the main reason for the high ratio of PtdIns(4)P to PtdIns(4,5)P2 found in terrestrial plants.
Our second goal was to increase the flux through the PI pathway to characterize the impact of increased PI signaling in a plant system. There were several advantages in using the human PtdIns(4)P 5-kinase, Hs PIPKIα, for increasing PtdIns(4,5)P2 in vivo: (1) Hs PIPKIα has a low Km for PtdIns(4)P and high Vmax compared with the plant kinases characterized thus far (Perera et al., 2005); (2) the preferred substrate is PtdIns(4)P, which is similar to plant lipid kinases (Westergren et al., 1999, 2001; Kunz et al., 2000, 2002; Perera et al., 2005); (3) Hs PIPKIα localized primarily with the plasma membrane in the tobacco cells, which means that it should directly affect the plasma membrane PtdIns(4,5)P2 pools; (4) readily detectable amounts of enzyme were produced in the transgenic cells unlike overexpression of At PIPK10; (5) Hs PIPKIα did not bind the actin cytoskeleton because the necessary human scaffolding proteins were not present in plants; (6) Hs PIPKIα does not contain MORN motifs so that additional effects of the MORN motifs found in At PIPK1-9 would not impact the interpretation of our results; and (7) by increasing the rate of one enzymatic reaction, we could alter the metabolic flux through the pathway.
We initially attempted to use At PIPK1 for this work; however, constitutive expression of At PIPK1 did not result in any detectable increase in functional protein or enzyme activity. We suspect that the inability to overproduce this enzyme in plants may result from the presence of the N-terminal MORN motifs or the actin binding domain in the linker region (Davis et al., 2007; Im et al., 2007). At PIPK10, which does not contain N-terminal MORN motifs or a linker region, can be overproduced. At PIPK10 increased PtdInsP2 biosynthesis and the flux through the pathway as predicted; however, the At PIPK10–induced changes in PI metabolism were marginal compared with those imparted by the more active enzyme Hs PIPKIα.
Tobacco cells expressing Hs PIPKIα had a 100-fold increase in their plasma membrane PtdInsP2 and a >40-fold increase in basal InsP3 based on mass assays. A combinatorial approach including in vitro characterization of plasma membrane enzyme activity and in vivo short- and long-term labeling studies indicated that the tobacco cells had sufficient PtdIns(4)P substrate to sustain a high rate of PtdIns(4,5)P2 biosynthesis. Furthermore, by increasing PtdIns(4,5)P2 biosynthesis, we increased total cellular Ins(1,4,5)P3. These data support our thesis that PtdInsP 5-kinase activity is a flux-limiting step in the plant PI pathway.
Profiling the plasma membrane lipids using electrospray ionization tandem mass spectrometry indicated that PtdOH levels were increased in the Hs PIPKIα lines. The simplest explanation for this increase is that diacylglycerol produced by PLC activity is phosphorylated by diacylglycerol kinase; however, we did not detect appreciable differences in PtdOH production with short-term [32P] labeling of whole cells. Alternatively, the increase in plasma membrane PtdOH in the Hs PIPKIα lines may result from increased PLD activity. Selective plant PLDs isoforms are activated by Ca2+ and PtdInsP2 (Pappan and Wang, 1999; Wang, 2000; Wang and Wang, 2001).
The impact of increased PtdIns(4,5)P2 biosynthesis was manifested in basal cellular metabolism. Previous work with animal cells indicated that PtdIns(4,5)P2 biosynthesis was completely inhibited by adding antimycin A and other inhibitors of mitochondrial respiration (Poggioli et al., 1983; Yeung et al., 2006). Changes in cytosolic calcium can directly affect mitochondria and stimulate respiration (McCormack et al., 1990; McCormack and Denton, 1993). It has been estimated that mitochondria can store up to 60% of the cellular calcium in some plant cells (Subbaiah et al., 1998; Logan and Knight, 2003). These data made a compelling argument that increasing PtdIns(4,5)P2 turnover might put a significant demand on ATP biosynthesis. The increased use of sugar from the culture medium and increased oxygen uptake in the Hs PIPKIα lines in conjunction with these previous studies in animal cells suggest that the status of PtdIns(4,5)P2 biosynthesis reflects the energy status of the cell. We also consistently observed a 30% increase in the plasma membrane vanadate-sensitive ATPase activity (data not shown). Taken together, the data indicate that when stimuli induce an increase in the PI pathway in plants, there will be an increased demand for ATP as the system responds and then recovers the PI pools to a prestimulated state.
If Ins(1,4,5)P3 increases cytosolic calcium in plants as predicted based on early microinjection experiments (Gilroy et al., 1990; Tucker and Boss, 1996), then there must be a continuous and rapid transport of Ca2+ out of the cells or into intracellular stores to retain homeostasis in the Hs PIPKIα lines. We observed an increase in ER calcium binding proteins, CRT and BiP, in the Hs PIPKIα lines, indicating that the ER is sensing a change in calcium homeostasis. Furthermore, the fact that the Hs PIPKIα lines required more extracellular Ca2+ to survive and that the ICP measurements indicated less total Ca2+/g dry weight suggests that the intracellular stores were depleted (Wu et al., 2002; Cheng et al., 2005). We cannot rule out the possibility that cell wall composition and therefore Ca2+ binding capacity is also compromised in the Hs PIPKIα lines. The fra3 mutants, which have increased PtdIns(4,5)P2 resulting from a mutation in type II InsP 5-ptase, had thinner cell walls (Zhong et al., 2004).
What is the expected consequence of increasing the plasma membrane PtdIns(4,5)P2 100-fold? A large increase in negative charge on the inner leaflet of the plasma membrane would be expected to affect lipid and protein interactions and alter cell structure and membrane biogenesis (Yeung et al., 2006). Increasing plasma membrane PtdIns(4,5)P2 has also been shown to generate actin comet tails and stress fibers and disrupt membrane trafficking in some animal cells (Rozelle et al., 2000; Yamamoto and Kiss, 2002; Kanzaki et al., 2004). We did not detect measurable differences in F-actin morphology based on phalloidin staining in the tobacco cells (see Supplemental Figure 5 online). This may be because the actin binding proteins required to scaffold Hs PIPKIα to actin were not present in the plant cells (Ridley, 2006; Davis et al., 2007). The intensity of phalloidin staining appeared to be consistently greater in the Hs PIPKIα lines, suggesting increased membrane permeability to phalloidin or possibly an increase in cable thickness reminiscent of the Arabidopsis fra3 mutant. However, even though actin filaments in the fra3 mutant were thickened and somewhat distorted, there was no evidence of actin comet tails (Zhong et al., 2004). Taken together with our observations that the plasma membrane PtdIns(4,5)P2 was increased even higher in the Hs PIPKIα lines than in the fra3 mutants, these data suggest that PtdIns(4,5)P2-mediated regulation of the actin cytoskeleton may be different in plant and animal cells (Davis et al., 2007).
The impact of increasing the flux through the pathway by increasing the rate PtdIns(4,5)P2 biosynthesis is understandably different from mutating, silencing, or knocking out a selective gene in the PI pathway (Burnette et al., 2003; Hunt et al., 2003; Zhong et al., 2004; Williams et al., 2005). Manipulating PI metabolism/turnover/signaling at different steps in the PI pathway could lead to different physiological consequences. In previous work (Perera et al., 2002, 2006), we showed that dampening the Ins(1,4,5)P3 signal and altering the flux by pulling metabolites through the PI pathway did not affect normal plant growth under optimal conditions but rather delayed and reduced gravitropic bending in Arabidopsis, which is consistent with the dampened signal (Perera et al., 2006). The data also indicate that plants have compensatory mechanisms for sensing environmental cues and that InsP3-mediated signaling can contribute to ∼30% of the normal gravity signal. By overexpressing Hs PIPKIα in this work, we have effectively pushed metabolites through the PI pathway and created plant cells that in theory should have reached a new steady state that is more similar to the rate of PI metabolism found in stimulated plant cells with InsP3 providing a greater portion of the input signal.
We show that fundamental metabolic pathways were altered in the Hs PIPKIα cells, including sugar use, respiration, and calcium homeostasis. These results emphasize the need to consider the impact of the turnover of second messengers on basal metabolism. Both transient or prolonged increases (15 min to several hours) in Ins(1,4,5)P3 have been documented in response to gravistimulation and osmotic stress (Perera et al., 1999; DeWald et al., 2001) and may have important downstream consequences (such as different Ca2+ signatures). The generation of constitutively stimulated plant cells provides a unique system for characterizing the physiological impact of PI signaling in plants.
The synthetic model system presented here provides some insights as to the consequences of PI signaling in a stimulated plant cell. Taken together with previously published data (Perera et al., 2002, 2005, 2006; Im et al., 2007), this work demonstrates that in normal plant cells, PIPK activity is flux limiting. To understand the system and eventually develop a predictive model, it is important to appreciate the flux-limiting step and the consequences of altering plant PI metabolism.
METHODS
Plant Material and Growth
Tobacco cells (Nicotiana tabacum NT-1 cells) were maintained in 25 mL of liquid culture medium as described previously (Perera et al., 2002) and subcultured weekly with a 6% (v/v) inoculum. To monitor cell growth, two replicate 25-mL cultures grown in 125-mL Erlenmeyer flasks at 125 rpm and 27°C were harvested at 2, 4, and 6 d after transfer. The cells were collected by low-speed centrifugation (∼500g for 3 min), and the fresh weight was measured. Cells were used 4 d after transfer unless otherwise indicated, and the average fresh weight harvested for both the wild-type and transgenic tobacco lines was 1.6 ± 0.2 g. For growth experiments with no added Ca2+, the tobacco culture media was made up with Murashige and Skoog salts except for the omission of Ca2+ salts.
Plant Transformation and Selection of Transgenic Lines
The cDNA encoding the At PIPK10 (At4g01190) and human PIPKIα (NM_003557) were subcloned into the pENTR/SD/D-TOPO destination vectors (Invitrogen) and then into pK7WGF2 (Functional Genomics Division of the Department of Plant Systems Biology, Gent, Belgium) for production of GFP fusion PIPK proteins under the control of a cauliflower mosaic virus 35S promoter in plants by LR recombination reaction according to the manufacturer's instructions (Invitrogen). The orientation of the resulting plasmids, pK7WGF2-PIPKs, was verified by PCR and DNA sequencing.
The recombinant binary plasmids (pK7WGF2-At PIPK10, pK7WGF2-Hs PIPKIα, and vector control pK7WGF2) were transformed into Agrobacterium tumefaciens EHA105 by the freeze-thaw method (Chen et al., 1994). NT-1 cells were transformed using Agrobacterium-mediated gene transfer as described (Perera et al., 2002). For each transformation, three independent, kanamycin-resistant microcalli were selected, and suspension cultures were established and maintained by weekly subculture in NT-1 medium containing 50 μg mL−1 kanamycin.
RNA Extraction and RT-PCR Analysis
To verify transformation and determine if the transgene was expressed, three independent transformed NT-1 lines/constructs were harvested after 4 d of growth and frozen in liquid N2. RNA was isolated using the plant RNeasy kit (Qiagen), with an additional DNase treatment to remove contaminating genomic DNA. Reverse transcription was performed using Omniscript reverse transcriptase enzyme according to the manufacturer's instructions (Qiagen). GFP-PIPK transcripts were detected by PCR using a forward primer to GFP and PIPK-specific reverse primers. PCR with actin-specific primers was performed to verify that an equal amount of template was used. PCR products were analyzed by gel electrophoresis.
Microscopy
Confocal fluorescence images were acquired with a Leica TCS SP1 confocal system using a Leica DM IRBE microscope and a ×40 numerical aperture 1.2 oil immersion objective. For GFP visualization, samples were excited with an argon laser at 488 nm, and fluorescence emission was collected from 500 to 560 nm. To visualize mitochondria, cells were stained with Rhodamine 123. To visualize actin, cells were fixed and stained with rhodamine phalloidin and visualized as described by Van Gestel et al. (2002).
Preparation of Microsomal and Plasma Membrane Fractions
Four-day-old NT-1 cells were harvested by filtration and immediately homogenized in 3 volumes of cold buffer as described (Perera et al., 2002), and the crude extract was clarified by centrifugation (5000g for 10 min at 4°C) to yield total cell lysate or fractionated further (40,000g, for 60 min, at 4°C) to yield microsomal and soluble protein fractions. Plasma membrane–enriched fractions were prepared from microsomes by aqueous two-phase partitioning as described previously (Perera et al., 1999). For enzyme assays, membrane fractions were placed on ice and assayed immediately. Protein concentrations were estimated using the Bio-Rad protein assay reagent with BSA as a standard.
Isolation of F-Actin from Plasma Membrane
F-actin was isolated from equal amounts of plasma membrane protein as described previously using a 20,000g centrifugation to recover large actin filaments and bundles (Stevenson-Paulik et al., 2003). After two rounds of polymerization, the 20,000g F-actin pellet was resuspended to a volume equal to the supernatant, and 20 μL of supernatant and pellet were assayed for PtdInsP 5-kinase activity as described below.
Immunoblotting
Immunoblotting was performed by SDS-PAGE of isolated proteins and transfered to a polyvinylidene difluoride membrane by electroblotting. Membranes were blocked with 3% (w/v) BSA, incubated with antibodies (anti-mouse GFP [Clontech] or anti-rabbit BiP and CRT), and incubated with horseradish peroxidase–conjugated anti-mouse or anti-rabbit. Immunoreactivity was visualized by incubating the blot in SuperSignal West Pico Chemiluminescent substrate (Pierce) and exposure to x-ray film. After chemiluminescence detection, total protein was visualized by staining the blots with Amido black (Sigma-Aldrich).
PtdInsP 5-Kinase Assays
In vitro lipid kinase assays were performed using 2 μg of plasma membrane protein. The standard assay was as previously described (Perera et al., 2002) with the following modifications. Reactions were performed either in the absence or presence of substrate [125 μM PtdIns(4)P from porcine brain; Avanti Polar Lipids) at room temperature for 10 min in a total volume of 50 μL. After incubation, phospholipids were extracted and separated by TLC as described (Perera et al., 2002).
Ins(1,4,5)P3 Assays and PtdIns(4,5)P2 Mass Measurements
Cells were harvested by filtration and immediately frozen in liquid N2, ground to a fine powder, and precipitated with cold 10% (v/v) perchloric acid (PCA). Ins(1,4,5)P3 assays were performed using the TRK1000 Ins(1,4,5)P3 assay kit (Amersham Pharmacia Biotech) as previously described (Perera et al., 1999, 2002), and PtdIns(4,5)P2 mass measurements were performed as described (Heilmann et al., 2001).
Lipid Profiling
To determine the effects of Hs PIPKIα expression on the plasma membrane lipid profile, we isolated plasma membrane–enriched fractions from 4-d-old wild-type and tobacco cells as described above. Lipid extraction, lipid analysis, and lipid quantification were performed as described (Welti et al., 2002) at the Kansas Lipidomics Facility.
In Vivo Labeling of Cells
In vivo labeling was performed with cells growing at the same rate and that had equivalent fresh weights. For 24-h labeling studies, 5 mL of cultures of 3-d-old wild-type and transgenic cells (∼0.1 g cells mL−1) were labeled with 25 μCi myo-[2-3H] inositol (25 Ci mmol−1). After 24 h, cells were harvested by filtration, ground in liquid N2, and incubated with cold 5% (v/v) PCA for 15 min on ice. The pellet and supernatant were separated by centrifugation, the pellet was washed with cold water twice, and the lipids were extracted as described previously (Perera et al., 2002). Extracted lipids were separated by TLC and quantified using a Bioscan imaging scanner or further processed for head group analysis. The phospholipids were deacylated for HPLC analysis as previously described (Hama et al., 2004). For soluble inositol phosphate analysis, cells were labeled as described above for 18 h and precipitated with PCA. The supernatant was analyzed by HPLC for soluble inositol phosphates using a Partisphere SAX strong anion exchange column as described by Stevenson-Paulik et al. (2006).
For short-term labeling, 4-d-old cells were harvested by filtration, weighed, preequilibrated in conditioned medium (0.2 g mL−1), and maintained by shaking. Conditioned medium was pooled from all the cell lines. After a 30-min recovery period, cells were labeled with carrier-free [32P] Pi (100 μCi mL−1). Equal aliquots (500 μL) of cells were removed at the indicated time points and added immediately to 500 μL of cold 20% (v/v) PCA and incubated on ice for ∼20 min. The pellet was washed with cold water twice, and lipids were extracted, separated by TLC, and quantified as described above.
O2 Uptake
Tobacco cells (4 d after transfer) were collected by filtration and preequilibrated in fresh medium at 0.1 g fresh weight mL−1 for 2 h on a rotary shaker (125 rpm, 25°C). Two-milliliter aliquots were used for measuring the rate of O2 uptake in a Clarke-type oxygen electrode system from Hansatech Instrument. The O2 concentration in the solution was measured over time at 25°C and recorded by an Omnitracer recorder (Houston Instruments).
ATPase Activity
Plasma membrane ATPase activity was determined as previously described (Wheeler and Boss, 1987) with and without the addition of V2O2. The vanadate-sensitive ATP hydrolysis is reported.
Calcium Uptake and Release Measurements
Ca2+ uptake and release were measured with ER-enriched microsomal membrane fraction as previously described (Persson et al., 2001). Ca2+ transport was measured with and without the addition of 3 mM ATP. Ca2+ release was determined from [45Ca2+]-loaded (23 min) vesicles by adding 1.5 μM ionomycin ionophore, and membrane vesicles were analyzed for [45Ca2+] after 5 min. The net release was determined as difference of [45Ca2+] recovered after the addition of ionomycin versus addition of dimethyl sulfoxide (same concentration as in the ionomycin stock) alone.
Whole-Cell Calcium Measurements
Cells (4 and 6 d old) were collected by filtration, washed twice with 5 mL of deionized water, and freeze-dried. The samples were dry ashed at 500°C overnight and digested in 6 n HCl according to Gorsuch (1970), and total calcium was determined using ICP emission spectrometry at the North Carolina State University Analytical Services Laboratory under the direction of Wayne Robarge.
Sugar Analysis
The sugar remaining in the culture medium was measured at the indicated day after transfer using Anthrone reagent as described by Van Handel (1985).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At4g01190 (Arabidopsis PIPK10) and NM_003557 (human PIPKIα).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Video 1. A Movie Showing the Localization of GFP-Hs PIPKIα with the Plasma Membranes of Tobacco Cells in Normal Medium.
Supplemental Video 2. A Movie Showing the Localization of GFP-Hs PIPKIα with the Plasma Membranes of Tobacco Cells after the Addition of 0.6 Osmolal Sorbitol.
Supplemental Figure 1. Head Group Analysis of PtdIns(4)P and PtdIns(4,5)P2 from the GFP and Hs PIPKIα Cell Lines.
Supplemental Figure 2. Calcium Uptake into the Mitochondria and Whole Cells.
Supplemental Figure 3. Ratiometric Imaging of Intracellular Calcium Using the Ca2+ Indicator, Indo-1.
Supplemental Figure 4. Visualization of Mitochondria with Rhodamine 123.
Supplemental Figure 5. Visualization of Actin with Rhodamine-Phalloidin.
Supplementary Material
Acknowledgments
We thank Richard Anderson (University of Wisconsin) for the gift of the human type Iα PIPK, Rebecca S. Boston (North Carolina State University) for the antibodies to CRT and BiP, and Wayne P. Robarge (North Carolina State University) for ICP analysis of calcium. This work was supported in part by funding from the North Carolina Agricultural Research Service (W.F.B. and N.S.A.), the National Science Foundation (W.F.B.), and by the Binational Science Foundation (W.F.B. and Nava Moran). The Kansas Lipidomics Research Center was supported by National Science Foundation Grants MCB0455318 and DBI 0521587, and National Science Foundation EPSCoR Grant EPS-0236913 with matching support from the State of Kansas through Kansas Technology Enterprise Corporation and Kansas State University. The Kansas Lipidomics Research Center is also supported by K-INBRE (National Institutes of Health Grant P20 RR16475 from the INBRE program of the National Center for Research Resources).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Wendy F. Boss (wendy_boss@ncsu.edu).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Adel-Latif, A.A., Smith, J.P., and Akhtar, R.A. (1985). Polyphosphoinositides and muscarinic chloinergic and β-adrenergic receptors in iris smooth muscle. In Inositol and Phosphoinositides: Metabolism and Regulation, J.E. Bleasdale, J. Eichberg, and G. Hauser, eds (Clifton, NJ: Humana Press), pp. 275–298.
- Akesson, A., Persson, S., Love, J., Boss, W.F., Widell, S., and Sommarin, M. (2005). Overexpression of the Ca2+-binding protein calreticulin in the endoplasmic reticulum improves growth of tobacco cell suspensions (Nicotiana tabacum) in high-Ca2+ medium. Physiol. Plant 123 92–99. [Google Scholar]
- Berdy, S.E., Kudla, J., Gruissem, W., and Gillaspy, G.E. (2001). Molecular characterization of At5PTase1, an inositol phosphatase capable of terminating inositol trisphosphate signaling. Plant Physiol. 126 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brearley, C.A., and Hanke, D.E. (1996). Metabolic evidence for the order of addition of individual phosphate esters to the myo-inositol moiety of inositol hexakisphosphate in the duckweed Spirodela polyrhiza L. Biochem. J. 314 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette, R.N., Gunesekera, B.M., and Gillaspy, G.E. (2003). An Arabidopsis inositol 5-phosphatase gain-of-function alters abscisic acid signaling. Plant Physiol. 132 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Nelson, R.S., and Sherwood, J.L. (1994). Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques 16 664–668, 670. [PubMed] [Google Scholar]
- Cheng, N.-H., Pittman, J.K., Shigaki, T., Lachmansingh, J., LeClere, S., Lahner, B., Salt, D.E., and Hirschi, K.D. (2005). Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 138 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, E., Thomas, G.M., Ball, A., Hiles, I., and Cockcroft, S. (1995). Phosphatidylinositol transfer protein dictates the rate of inositol trisphosphate production by promoting the synthesis of PIP2. Curr. Biol. 5 775–783. [DOI] [PubMed] [Google Scholar]
- Davis, A.J., Im, Y.J., Dubin, J.S., Tomer, K.B., and Boss, W.F. (2007). Arabidopsis phosphatidylinositol phosphate kinase 1 binds F-actin and recruits phosphatidylinositol 4-kinase beta 1 to the actin cytoskeleton. J. Biol. Chem. 282 14121–14131. [DOI] [PubMed] [Google Scholar]
- DeWald, D.B., Torabinejad, J., Jones, C.A., Shope, J.C., Cangelosi, A.R., Thompson, J.E., Prestwich, G.D., and Hama, H. (2001). Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed Arabidopsis. Plant Physiol. 126 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, P.E., Coursol, S., Skirpan, A.L., Kao, T.H., and Gilroy, S. (2006). Petunia phospholipase C1 is involved in pollen tube growth. Plant Cell 18 1438–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drøbak, B.K., Dewey, R.E., and Boss, W.F. (1999). Phosphoinositide kinases and the synthesis of polyphosphoinositides in higher plant cells. In International Review of Cytology, K.W. Jeon, ed (New York: Academic Press), pp. 95–130. [DOI] [PubMed]
- Elge, S., Brearley, C., Xia, H.J., Kehr, J., Xue, H.W., and Mueller-Roeber, B. (2001). An Arabidopsis inositol phospholipid kinase strongly expressed in procambial cells: Synthesis of Ptdlns(4,5)P2 and Ptdlns(3,4,5)P3 in insect cells by 5-phosphorylation of precursors. Plant J. 26 561–571. [DOI] [PubMed] [Google Scholar]
- Ercetin, M.E., and Gillaspy, G.E. (2004). Molecular characterization of an Arabidopsis gene encoding a phospholipid-specific inositol polyphosphate 5-phosphatase. Plant Physiol. 135 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy, S., Read, N.D., and Trewavas, A.J. (1990). Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature 346 769–771. [DOI] [PubMed] [Google Scholar]
- Gorsuch, T.T. (1970). The Destruction of Organic Matter. (Elmsford, NY: Pergamon Press).
- Gross, W., and Boss, W.F. (1993). Inositol phospholipids and signal transduction. In Control of Plant Gene Expression, D.P.S. Verma, ed (Boca Raton, FL: CRC Press), pp. 17–32.
- Hama, H., Torebinejad, J., Prestwich, G., and DeWald, D. (2004). Measurement and immunofluorescence of cellular phosphoinositides. In Methods in Molecular Biology: Signal Transduction Protocols, R. Dickson, ed (Clifton, NJ: Humana Press), pp. 243–258. [DOI] [PubMed]
- Haseloff, J., and Amos, B. (1995). GFP in plants. Trends Genet. 11 328–329. [DOI] [PubMed] [Google Scholar]
- Heilmann, I., Perera, I.Y., Gross, W., and Boss, W.F. (2001). Plasma membrane phosphatidylinositol 4,5-bisphosphate decreases with time in culture. Plant Physiol. 126 1507–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling, D., Possart, A., Cottier, S., Klahre, U., and Kost, B. (2006). Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell 18 3519–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, L., Mills, L.N., Pical, C., Leckie, C.P., Aitken, F.L., Kopka, J., Mueller-Roeber, B., McAinsh, M.R., Hetherington, A.M., and Gray, J.E. (2003). Phospholipase C is required for the control of stomatal aperture by ABA. Plant J. 34 47–55. [DOI] [PubMed] [Google Scholar]
- Im, Y.J., Davis, A.J., Perera, I.Y., Johannes, E., Allen, N.S., and Boss, W.F. (2007). The N-terminal membrane occupation and recognition nexus domain of Arabidopsis phosphatidylinositol phosphate kinase 1 regulates enzyme activity. J. Biol. Chem. 282 5443–5452. [DOI] [PubMed] [Google Scholar]
- Kanzaki, M., Furukawa, M., Raab, W., and Pessin, J.E. (2004). Phosphatidylinositol 4,5-bisphosphate regulates adipocyte actin dynamics and GLUT4 vesicle recycling. J. Biol. Chem. 279 30622–30633. [DOI] [PubMed] [Google Scholar]
- Kunz, J., Fuelling, A., Kolbe, L., and Anderson, R.A. (2002). Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a single amino acid residue. J. Biol. Chem. 277 5611–5619. [DOI] [PubMed] [Google Scholar]
- Kunz, J., Wilson, M.P., Kisseleva, M., Hurley, J.H., Majerus, P.W., and Anderson, R.A. (2000). The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol. Cell 5 1–11. [DOI] [PubMed] [Google Scholar]
- Lievremont, J.P., Rizzuto, R., Hendershot, L., and Meldolesi, J. (1997). BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+. J. Biol. Chem. 272 30873–30879. [DOI] [PubMed] [Google Scholar]
- Logan, D.C., and Knight, M.R. (2003). Mitochondrial and cytosolic calcium dynamics are differentially regulated in plants. Plant Physiol. 133 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, Y., Gou, J.-Y., and Xue, H.-W. (2007). PIP5K9, an Arabidopsis phosphatidylinositol monophosphate kinase, interacts with a cytosolic invertase to negatively regulate sugar-mediated root growth. Plant Cell 19 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack, J.G., and Denton, R.M. (1993). Mitochondrial Ca2+ transport and the role of intramitochondrial Ca2+ in the regulation of energy metabolism. Dev. Neurosci. 15 165–173. [DOI] [PubMed] [Google Scholar]
- McCormack, J.G., Halestrap, A.P., and Denton, R.M. (1990). Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70 391–425. [DOI] [PubMed] [Google Scholar]
- Meijer, H.J.G., and Munnik, T. (2003). Phospholipid-based signaling in plants. Annu. Rev. Plant Biol. 54 265–306. [DOI] [PubMed] [Google Scholar]
- Michalak, M., Corbet, E.F., Mesaeli, N., Nakamura, K., and Opas, M. (1999). Calreticulin: One protein, one gene, many functions. Biochem. J. 344 281–292. [PMC free article] [PubMed] [Google Scholar]
- Mikami, K., Katagiri, T., Iuchi, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1998). A gene encoding phosphatidylinositol-4-phosphate 5-kinase is induced by water stress and abscisic acid in Arabidopsis thaliana. Plant J. 15 563–568. [DOI] [PubMed] [Google Scholar]
- Mueller-Roeber, B., and Pical, C. (2002). Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol. 130 22–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik, T., Irvine, R.F., and Musgrave, A. (1998). Phospholipid signalling in plants. Biochim. Biophys. Acta 1389 222–272. [DOI] [PubMed] [Google Scholar]
- Pappan, K., and Wang, X. (1999). Plant phospholipase Dα is an acidic phospholipase active at near-physiological Ca(2+) concentrations. Arch. Biochem. Biophys. 368 347–353. [DOI] [PubMed] [Google Scholar]
- Perera, I.Y., Davis, A.J., Galanopoulou, D., Im, Y.J., and Boss, W.F. (2005). Characterization and comparative analysis of Arabidopsis phosphatidylinositol phosphate 5-kinase 10 reveals differences in Arabidopsis and human phosphatidylinositol phosphate kinases. FEBS Lett. 579 3427–3432. [DOI] [PubMed] [Google Scholar]
- Perera, I.Y., Heilmann, I., and Boss, W.F. (1999). Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc. Natl. Acad. Sci. USA 96 5838–5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, I.Y., Hung, C.Y., Brady, S., Muday, G.K., and Boss, W.F. (2006). A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol. 140 746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, I.Y., Love, J., Heilmann, I., Thompson, W.F., and Boss, W.F. (2002). Up-regulation of phosphoinositide metabolism in tobacco cells constitutively expressing the human type I inositol polyphosphate 5-phosphatase. Plant Physiol. 129 1795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, S., Love, J., Tsou, P.-L., Robertson, D., Thompson, W.F., and Boss, W.F. (2002). When a day makes a difference. Interpreting data from endoplasmic reticulum-targeted green fluorescent protein fusions in cells grown in suspension culture. Plant Physiol. 128 341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, S., Wyatt, S.E., Love, J., Thompson, W.F., Robertson, D., and Boss, W.F. (2001). The Ca2+ status of the endoplasmic reticulum is altered by induction of calreticulin expression in transgenic plants. Plant Physiol. 126 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillippy, B.Q. (1998). Identification of inositol 1,3,4-trisphosphate 5-kinase and inositol 1,3,4,5-tetrakisphosphate 6-kinase in immature soybean seeds. Plant Physiol. 116 291–297. [Google Scholar]
- Pical, C., Westergren, T., Dove, S.K., Larsson, C., and Sommarin, M. (1999). Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4,5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J. Biol. Chem. 274 38232–38240. [DOI] [PubMed] [Google Scholar]
- Poggioli, J., Weiss, S.J., McKinney, J.S., and Putney, J.W., Jr. (1983). Effects of antimycin A on receptor-activated calcium mobilization and phosphoinositide metabolism in rat parotid gland. Mol. Pharmacol. 23 71–77. [PubMed] [Google Scholar]
- Preuss, M.L., Schmitz, A.J., Thole, J.M., Bonner, H.K., Otegui, M.S., and Nielsen, E. (2006). A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J. Cell Biol. 172 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboy, V. (2001). Seeds for a better future: ‘Low phytate’ grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 6 458–462. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J. (2006). Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16 522–529. [DOI] [PubMed] [Google Scholar]
- Rozelle, A.L., Machesky, L.M., Yamamoto, M., Driessens, M.H., Insall, R.H., Roth, M.G., Luby-Phelps, K., Marriott, G., Hall, A., and Yin, H.L. (2000). Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr. Biol. 10 311–320. [DOI] [PubMed] [Google Scholar]
- Sandelius, A.S., and Sommarin, M. (1990). Membrane-localized reactions involved in polyphosphoinositide turnover in plants. In Inositol Metabolism in Plants, D.J. Morre, W.F. Boss, and F.A. Loewus, eds (New York: Wiley-Liss), pp. 139–161.
- Stevenson-Paulik, J., Chiou, S.T., Frederick, J.P., Cruz, J.D., Seeds, A.M., Otto, J.C., and York, J.D. (2006). Inositol phosphate metabolomics: Merging genetic perturbation with modernized radiolabeling methods. Methods 39 112–121. [DOI] [PubMed] [Google Scholar]
- Stevenson-Paulik, J., Love, J., and Boss, W.F. (2003). Differential regulation of two Arabidopsis type III phosphatidylinositol 4-kinase isoforms. A regulatory role for the pleckstrin homology domain. Plant Physiol. 132 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson-Paulik, J., Odom, A.R., and York, J.D. (2002). Molecular and biochemical characterization of two plant inositol polyphosphate 6-/3-/5-kinases. J. Biol. Chem. 277 42711–42718. [DOI] [PubMed] [Google Scholar]
- Stevenson, J.M., Perera, I.Y., and Boss, W.F. (1998). A phosphatidylinositol 4-kinase pleckstrin homology domain that binds phosphatidylinositol 4-monophosphate. J. Biol. Chem. 273 22761–22767. [DOI] [PubMed] [Google Scholar]
- Stevenson, J.M., Perera, I.Y., Heilmann, I., Persson, S., and Boss, W.F. (2000). Inositol signaling and plant growth. Trends Plant Sci. 5 252–258. [DOI] [PubMed] [Google Scholar]
- Subbaiah, C.C., Bush, D.S., and Sachs, M.M. (1998). Mitochondrial contribution to the anoxic Ca2+ signal in maize suspension-cultured cells. Plant Physiol. 118 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Z., and Boss, W.F. (1992). Association of phosphatidylinositol kinase, phosphatidylinositol monophosphate kinase, and diacylglycerol kinase with the cytoskeleton and F-actin fractions of carrot (Daucus carota L.) cells grown in suspension culture. Plant Physiol. 100 2116–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, E.B., and Boss, W.F. (1996). Mastoparan-induced intracellular Ca2+ fluxes may regulate cell-to-cell communication in plants. Plant Physiol. 111 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gestel, K., Kohler, R.H., and Verbelen, J.P. (2002). Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. J. Exp. Bot. 53 659–667. [DOI] [PubMed] [Google Scholar]
- Van Handel, E. (1985). Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control Assoc. 1 299–301. [PubMed] [Google Scholar]
- Van Leeuwen, W., Okresz, L., Bogre, L., and Munnik, T. (2004). Learning the lipid language of plant signalling. Trends Plant Sci. 9 378–384. [DOI] [PubMed] [Google Scholar]
- Verma, D.P.S., and Maclachlan, G.A. (1976). Metabolism of poly(A) in plant cells: Discrete classes associated with free and membrane-bound polysomes. Plant Physiol. 58 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, P., Chua, M., Nogue, F., Fairbrother, A., Mekeel, H., Xu, Y., Allen, N., Bibikova, T.N., Gilroy, S., and Bankaitis, V.A. (2005). A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J. Cell Biol. 168 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., and Wang, X. (2001). A novel phospholipase D of Arabidopsis that is activated by oleic acid and associated with the plasma membrane. Plant Physiol. 127 1102–1112. [PMC free article] [PubMed] [Google Scholar]
- Wang, X. (2000). Multiple forms of phospholipase D in plants: The gene family, catalytic and regulatory properties, and cellular functions. Prog. Lipid Res. 39 109–149. [DOI] [PubMed] [Google Scholar]
- Welti, R., Li, W., Li, M., Sang, Y., Biesiada, H., Zhou, H.-E., Rajashekar, C.B., Williams, T.D., and Wang, X. (2002). Profiling membrane lipids in plant stress responses. Role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 277 31994–32002. [DOI] [PubMed] [Google Scholar]
- Westergren, T., Dove, S.K., Sommarin, M., and Pical, C. (2001). AtPIP5K1, an Arabidopsis thaliana phosphatidylinositol phosphate kinase, synthesizes PtdIns(3,4)P2 and PtdIns(4,5)P2 in vitro and is inhibited by phosphorylation. Biochem. J. 359 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren, T., Ekblad, L., Jergil, B., and Sommarin, M. (1999). Phosphatidylinositol 4-kinase associated with spinach plasma membranes. Isolation and characterization of two distinct forms. Plant Physiol. 121 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, J.J., and Boss, W.F. (1987). Polyphosphoinositides are present in plasma membranes isolated from fusogenic carrot cells. Plant Physiol. 85 389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M.E., Torabinejad, J., Cohick, E., Parker, K., Drake, E.J., Thompson, J.E., Hortter, M., and Dewald, D.B. (2005). Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress-response pathway. Plant Physiol. 138 686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., Liang, F., Hong, B., Young, J.C., Sussman, M.R., Harper, J.F., and Sze, H. (2002). An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol. 130 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, K., and Kiss, J.Z. (2002). Disruption of the actin cytoskeleton results in the promotion of gravitropism in inflorescence stems and hypocotyls of Arabidopsis. Plant Physiol. 128 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, T., Terebiznik, M., Yu, L., Silvius, J., Abidi, W.M., Philips, M., Levine, T., Kapus, A., and Grinstein, S. (2006). Receptor activation alters inner surface potential during phagocytosis. Science 313 347–351. [DOI] [PubMed] [Google Scholar]
- Zhong, R., Burk, D.H., Morrison III, W.H., and Ye, Z.H. (2004). FRAGILE FIBER3, an Arabidopsis gene encoding a type II inositol polyphosphate 5-phosphatase, is required for secondary wall synthesis and actin organization in fiber cells. Plant Cell 16 3242–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.