Abstract
Phosphorus, one of the essential elements for plants, is often a limiting nutrient in soils. Low phosphate (Pi) availability induces sugar-dependent systemic expression of genes and modulates the root system architecture (RSA). Here, we present the differential effects of sucrose (Suc) and auxin on the Pi deficiency responses of the primary and lateral roots of Arabidopsis (Arabidopsis thaliana). Inhibition of primary root growth and loss of meristematic activity were evident in seedlings grown under Pi deficiency with or without Suc. Although auxin supplementation also inhibited primary root growth, loss of meristematic activity was observed specifically under Pi deficiency with or without Suc. The results suggested that Suc and auxin do not influence the mechanism involved in localized Pi sensing that regulates growth of the primary root and therefore delineates it from sugar-dependent systemic Pi starvation responses. However, the interaction between Pi and Suc was evident on the development of the lateral roots and root hairs in the seedlings grown under varying levels of Pi and Suc. Although the Pi+ Suc− condition suppressed lateral root development, induction of few laterals under the Pi− Suc− condition point to increased sensitivity of the roots to auxin during Pi deprivation. This was supported by expression analyses of DR5∷uidA, root basipetal transport assay of auxin, and RSA of the pgp19 mutant exhibiting reduced auxin transport. A significant increase in the number of lateral roots under the Pi− Suc− condition in the chalcone synthase mutant (tt4-2) indicated a potential role for flavonoids in auxin-mediated Pi deficiency-induced modulation of RSA. The study thus demonstrated differential roles of Suc and auxin in the developmental responses of ontogenetically distinct root traits during Pi deprivation. In addition, lack of cross talk between local and systemic Pi sensing as revealed by the seedlings grown under either the Pi− Suc− condition or in the heterogenous Pi environment highlighted the coexistence of Suc-independent and Suc-dependent regulatory mechanisms that constitute Pi starvation responses.
Phosphate (Pi) is a structural component of many metabolites and plays a pivotal role in energy balance and carbon assimilation. Whereas the total Pi in soil could be high, its availability to plants is regulated by the process of mineralization and/or by competitive microorganisms (for review, see Vance et al., 2003; Raghothama and Karthikeyan, 2005). Pi starvation-induced (PSI) changes in root system architecture (RSA) facilitate efficient mobilization and acquisition of Pi from the growth medium (Lynch, 1995; Forde, 2002). Low-Pi status of the plant triggers systemic adaptive responses, including spatiotemporal regulation of a large array of PSI genes (Burleigh and Harrison, 1999; Misson et al., 2005). Systemic regulation of gene expression by Pi deficiency was demonstrated using divided root studies (Burleigh and Harrison, 1999; Baldwin et al., 2001). In addition, localized Pi deprivation also serves as a signal that strongly influences root system (Linkohr et al., 2002). Likewise, localized supply of nitrate also influences root system responses in Arabidopsis (Arabidopsis thaliana; Zhang and Forde, 1998). These studies therefore suggested a potential role of localized nutrient availability on RSA. Cross talk between local and systemic Pi sensing has been postulated under homogenous Pi conditions (Sánchez-Calderón et al., 2006), but the interaction between them under a heterogenous Pi environment is not thoroughly understood.
Growing evidence suggests that sugars act as signaling intermediates in multiple pathways, including those involved in root system responses to environmental perturbations (Freixes et al., 2002; Rolland and Sheen, 2005). Cross talk between sugar sensing and systemic Pi starvation responses has been explicitly demonstrated in several studies (Franco-Zorrilla et al., 2005; Liu et al., 2005; Karthikeyan et al., 2007). It appears that lack of sugar suppresses the expression of a host of PSI genes. At present, little is known about the specific involvement of sugars in sensing of local availability of Pi and consequent developmental responses of ontogenetically distinct RSA traits.
Pi deficiency results in a drastic reduction in primary root growth and alterations in lateral root formation (Williamson et al., 2001; López-Bucio et al., 2002, 2005). These changes in root architecture appear to be mediated at least in part by hormonal signaling. A complex interaction among cytokinin, sugar, and Pi starvation signaling has been proposed (Franco-Zorrilla et al., 2005). However, cytokinin has largely been implicated in the systemic repression of PSI gene expression with no apparent effect on localized Pi-dependent root traits (Martín et al., 2000). Because auxin plays a significant role in root development (Reed et al., 1998; Casimiro et al., 2001; Bhalerao et al., 2002), the probable cross talk between Pi sensing and auxin signaling has been a subject of great interest (Williamson et al., 2001; López-Bucio et al., 2005; Nacry et al., 2005). Exogenous application of auxin to Pi-replete roots resulted in localized alterations in root architecture that mimicked those seen under Pi deficiency (Gilbert et al., 2000; López-Bucio et al., 2002). Increased auxin sensitivity of the roots of Pi-deficient Arabidopsis was linked to increased expression of the auxin-responsive reporter DR5∷uidA (Nacry et al., 2005). Although Pi deficiency-induced alterations in Arabidopsis root architecture have been described as auxin dependent (López-Bucio et al., 2002, 2005; Al-Ghazi et al., 2003; Nacry et al., 2005), there are studies suggesting the presence of an auxin-independent response as well (Williamson et al., 2001; Ticconi et al., 2004). The discrepancies in observed results may reflect growth conditions and/or intrinsic diversity within and across Arabidopsis accessions (Chevalier et al., 2003). This is particularly relevant because even isogenic seedlings have been shown to exhibit extensive variability in lateral root initiation (Malamy and Ryan, 2001). Furthermore, the interaction between sugar and auxin in mediating PSI responses has not been well elucidated.
In this study, we evaluated the interactions between Suc and auxin in mediating Pi deficiency responses on RSA of Arabidopsis. Primary root growth, meristematic activity, and auxin responsiveness of the seedlings showed that Suc and auxin may not play a role in the determinate growth of the primary root exposed to localized Pi deficiency. On the other hand, auxin transport assay and RSA analyses of the wild-type and auxin transport mutants suggest the involvement of both auxin and Suc in development of lateral roots during Pi deficiency. The study highlights the differential effects of Suc and auxin on Pi deficiency-induced modulations of ontogenetically distinct root traits.
RESULTS
Pi and Suc Availability Affect Morphophysiological Traits
The effects of Suc and Pi availability on the growth and development of Arabidopsis were evaluated by analyzing the morphophysiological traits of seedlings grown under Pi+ Suc+ (P+S+), Pi+ Suc− (P+S−), Pi− Suc+ (P−S+), and Pi− Suc− (P−S−) conditions for 7 d (Fig. 1). Pi+ and Pi− refer to 1.25 mm and 20 μm of Pi, respectively. Nutrient medium was supplemented with 1.5% Suc (Suc+) or no Suc (Suc−; see “Materials and Methods”). Relative to P+S+ and P−S+ seedlings, there was a significant reduction of about 40% to 45% in the fresh weight of P+S− and P−S− seedlings. Regardless of Pi status, there was a substantial effect of Suc availability on the fresh weight of the seedlings (Fig. 1A) and the interaction between Pi and Suc was not significant (P < 0.05) on this trait. Although the difference between the shoot-to-root ratio of P+S+ and P+S− seedlings was not significant (P < 0.05), a 40% reduction in the ratio was observed between P−S+ and P−S− seedlings, revealing a differential effect of Suc under P+ and P− conditions (Fig. 1B). Relative to P+S+ seedlings, the level of soluble Pi in P−S+ shoots and roots was drastically reduced by 60% and 82%, respectively. Comparatively, the reduction in Pi content was less pronounced between P+S− and P−S− shoots and roots (Fig. 1C). The total leaf area, an indicator of the amount of photosynthate available for biomass production (Muldoon et al., 1984), also showed significant reduction (P < 0.05) during deprivation of Suc and/or Pi (Fig. 1D). Furthermore, accumulation of anthocyanins in shoots, a typical response to Pi deficiency, was significantly higher (P < 0.05) in P−S+ seedlings as compared to seedlings grown under other nutrient conditions (Fig. 1E). The data thus revealed significant (P < 0.05) interaction between Pi and Suc on shoot-to-root ratio, leaf area, Pi, and anthocyanin content in the seedlings.
Figure 1.
Effect of Pi and Suc availability on morphophysiological traits. Wild-type seedlings were grown for 7 d on vertically oriented agar petri plates containing P+S+, P+S−, P−S+, or P−S− nutrient. Data are presented for fresh weight; n = 6 replicates of 20 seedlings each (A); shoot-to-root fresh weight biomass ratio, n = 6 replicates of 20 seedlings each (B); soluble Pi content, n = 6 replicates of 100 to 150 seedlings each (C); leaf area, n = 4, ▪ = 1 mm2 (D); and anthocyanin content, n = 6 replicates of 100 to 150 seedlings each (E). Values are means ± se. Different letters indicate that the means differ significantly (P < 0.05).
Transition of Primary Root to Determinate Growth under Pi Deficiency Does Not Require Suc
Localized Pi deficiency triggers progressive loss of meristematic cells in the primary root, thereby causing determinate growth (Sánchez-Calderón et al., 2005). We used transgenic plants expressing the cell cycle marker CycB1;1∷uidA (Ferreira et al., 1994) to investigate the temporal effects of Suc and/or Pi deprivation on primary root growth (Fig. 2A). Over a period of 2 d, P+S+ seedlings grew significantly (P < 0.05) more (1.3 cm) compared to the marginal increase (0.2–0.4 cm) in seedlings grown under other nutrient conditions. By the fourth day, there was an almost 2-fold increase in the primary root growth of P+S+ seedlings and a slight, but significant (P < 0.05), increase under P+S− conditions as well. This trend became more apparent at 7 d. Seedlings grown under P−S+ and P−S− showed only 0.6- to 0.8-cm increase in primary root length and the difference between these two treatments was not significant (P < 0.05; Fig. 2, A and B, top). Supplementation with Suc and Pi (P+S+) did not induce any significant (P < 0.05) growth of the primary root either in P−S+ or P−S− seedlings, whereas a significant increase (P < 0.05) in the length (4–5 cm) was observed in both P+S+ and P+S− seedlings (Fig. 2, A and B, bottom). Further, the role of Suc in Pi deficiency-induced alterations in meristematic activity was evaluated by comparing the expression of CycB1;1∷uidA in the primary root tips of seedlings grown under these nutrient conditions (Fig. 2C, top) and after replenishment with P+S+ (Fig. 2C, bottom). Before and after replenishment treatments, primary root tips of P+S+ and P+S− seedlings revealed strong expression of CycB1;1∷uidA, whereas it was barely detected in P−S+ and P−S− seedlings. Our study thus provided evidence that Pi deficiency-induced loss of meristematic activity in the primary root is independent of Suc availability. Analysis of the pldz2 mutant, defective in the hydrolysis of phospholipids and reduced capacity to accumulate galactolipids under Pi-limiting conditions, suggested the role of Pi deficiency-induced PLDZ2 in maintenance of the root system (Cruz-Ramírez et al., 2006). Because our data showed Suc-independent Pi deficiency-induced inhibition of primary growth, we investigated the role of Suc in mediating Pi deficiency-induced regulation of PLDZ2 (Fig. 2D). Real-time PCR analysis revealed a significant increase in the expression of PLDZ2 and the high-affinity Pi transporter Pht1;4 (used as a control) in P−S+ seedlings relative to P+S+ and P+S− seedlings. However, this increase in the expression of both the genes was substantially reduced when Pi-deprived plants were starved of Suc. This suggested the likely involvement of components other than PLDZ2 that trigger Pi deficiency-induced loss of meristematic activity of primary roots independent of Suc status in the nutrient medium.
Figure 2.
Role of Suc in Pi deficiency-induced determinate primary root growth. A, Transgenic CycB1;1∷uidA Arabidopsis seedlings, grown on different nutrient media, were scanned sequentially at indicated time intervals and after replenishment with P+S+ nutrient for temporal analysis of primary root growth. B, RSAs are representative of 20 seedlings each of P+S+ (a), P+S− (b), P−S+ (c), and P−S− (d). Lateral roots were spread under a stereomicroscope to present their architectural details. At the bottom (e–h), → indicates primary root length at the time of transfer to P+S+ for replenishment. Scale bar = 1 cm. C, Histochemical GUS staining of primary root tips of CycB1;1∷uidA seedlings grown for 7 d on P+S+ (a), P+S− (b), P−S+ (c), and P−S− (d) and after their replenishment (e–h) with P+S+ for 7 d. GUS-stained seedlings were observed under compound microscopy (20× objective). Photographs are representative of at least 10 to 12 seedlings. Scale bar = 50 μm. D, Real-time PCR analysis of the relative expression levels of Pht1;4 and PLDZ2 in seedlings grown on vertically oriented agar petri plates under different nutrient conditions for 7 d. 18S ribosomal RNA was used as an internal control. Data presented are the means of three technical replicates ± se. [See online article for color version of this figure.]
Determinate Primary Root Growth Induced by Pi Deficiency Is Independent of Auxin
It has been suggested that inhibition of primary root growth during Pi deprivation is auxin dependent (Nacry et al., 2005). We used DR5∷uidA to evaluate the effect of Pi and/or Suc deprivation on auxin-dependent transcription in primary root tips (Fig. 3). The extent of stained regions in primary root tips of the seedlings grown in Suc-enriched medium with or without Pi was more than those grown without Suc (Fig. 3A). This was consistent with the free indole acetic acid (IAA) levels observed in the lower portion (4 mm) of the roots of the corresponding seedlings (Fig. 3B). Free IAA accumulations in upper roots were less affected by Suc or Pi status (Fig. 3B). Root-bending assays revealed normal gravitropic response regardless of Suc or Pi status (data not shown). This suggested that Pi and Suc deficiency did not interrupt the minimal levels of basipetal auxin redirection at the root tip required for graviresponsive bending. In fact, basipetal auxin transport from root tips was 30%–70% significantly higher (P < 0.05) in 2- to 6-mm segments proximal to the root tip of P−S− seedlings as compared to those grown under other nutrient conditions (Fig. 3C). However, the observed differences in auxin accumulation and basipetal auxin transport did not correlate with variable root growth among different treatments (see Fig. 2B, top). Although addition of 0.1 μm IAA to nutrient media inhibited the primary root growth of CycB1;1∷uidA seedlings (Fig. 3D), expression of the marker∷uidA gene was maintained only in Pi-replete (P+S+ and P+S−) seedlings (Fig. 3E). When these seedlings were transferred to P+S+ medium, the primary roots of P+S+ and P+S− seedlings resumed normal growth, whereas those of P−S+ and P−S− seedlings remained determinant (Fig. 3F). This provided evidence for the auxin-independent effect of Pi deprivation on the determinate growth of the primary root.
Figure 3.
Effect of auxin availability on Pi deficiency-induced determinate growth of the primary root. A, Transgenic DR5∷uidA Arabidopsis seedlings were grown on different nutrient media for 7 d. GUS-stained hypocotyl-root junction and tips of primary and lateral roots were observed under compound microscopy (20× objective). Photographs are representative of eight to 10 seedlings for each of the treatments. Scale bar = 0.1 mm. B, Free IAA content in upper and lower roots (4 mm) of seedlings grown in different nutrient media for 7 d. Values are mean ± sd from 500 seedlings per replicate, n = 3. *, Means differ significantly (P < 0.05). C, Polar transport of [3H] IAA assay 2, 4, and 6 mm from the root tips of the seedlings grown under different nutrient conditions for 3 d. Values are means ± sd from 10 seedlings per replicate (n = 3). D, Representative RSAs of 20 seedlings each of transgenic CycB1;1∷uidA Arabidopsis seedlings grown for 7 d in 0.1 μm IAA-supplemented nutrient medium. •, Primary root length (average = 2.4 cm; n = 80) at the time of transfer to auxin-supplemented nutrient medium. Scale bar = 1 cm. E, Ten of the transgenic CycB1;1∷uidA seedlings for each of the treatments shown in D were used for histochemical GUS staining of the primary root tip. F, The rest of them were transferred to P+S+ for 7 d for replenishment. →, Primary root length achieved in auxin-supplemented different nutrient media at the time of replenishment. Some of the lateral roots growing near the root tip were removed to expose the primary root. Scale bars = 100 μm and 50 μm in A and E, respectively; 1 cm in D and F.
Pi Deficiency Promotes Suc-Mediated Initiation of Higher Order Laterals
Studies on the effects of Pi deprivation on lateral roots in Arabidopsis have led to contrasting inferences, implying either increase (López-Bucio et al., 2002) or decrease (Al-Ghazi et al., 2003; Nacry et al., 2005) in their numbers. Also, the role of Suc in mediating these responses is not well elucidated. We therefore investigated whether induction of determinate growth of the primary root during Pi deficiency coincides with initiation of new primary laterals or, alternatively, promotes development of higher order laterals (Fig. 4). Seedlings grown for 7 d under P+S+ conditions exhibited extensive variability in the number of primary laterals ranging from seven to 26 with an average of 13.7 (n = 20; sd = 5.8) and a similar trend was revealed under P−S+ conditions with the number varying from 10 to 21 with an average of 14 (n =20; sd = 3.2; Fig. 4A). Therefore, it is not surprising to find conflicting reports on the effect of Pi deprivation on the development of lateral roots. However, there was a significant increase (P < 0.05) in the number of second-order laterals in the P−S+ seedling compared to those grown under the P+S+ condition. The effect of Pi status on the number of primary laterals, however, became more distinct either during prolonged treatment (up to 14 d; Fig. 4B) or if nutrient (P+S+) was replenished after 7 d (Fig. 4C). Under these conditions, P+S+ seedlings exhibited an almost 2-fold increase in the number of primary and second-order laterals compared to what was seen at 7 d (Fig. 4, B and C). In contrast, no increase in primary laterals was seen in seedlings grown on P−S+ at 14 d or transferred to P+S+ at 7 d. However, a 2-fold increase in second-order laterals was seen under these conditions and a small number of the seedlings also produced third- and fourth-order laterals (Fig. 4, B and C). Interestingly, lateral root formation was largely inhibited under P+S− conditions with only occasional development of a few primary laterals mostly in the vicinity of the root-to-shoot junction (Fig. 2B, top). Seedlings grown under P−S− conditions initiated primary laterals closer to the root tips compared to those grown under P+S− conditions, suggesting a likely effect of Pi deprivation on the development of this trait (Fig. 2B, top). However, similar to P−S+ seedlings, P−S− seedlings did not show any significant increase (P < 0.05) in the number of primary laterals during prolonged growth or upon replenishment with P+S+ (Fig. 4, B and C). In addition, initiation of higher order laterals in P−S− seedlings was suppressed as compared to P−S+ seedlings (Fig. 4, B and C). The results, therefore, suggested a Pi deficiency-induced irreversible inhibitory effect on the initiation of the primary laterals and a shift to the formation of higher order laterals, which was accentuated by Suc availability. On the contrary, Suc deprivation-induced inhibition of lateral roots in P+S− seedlings (Fig. 4A) could be alleviated upon replenishment with P+S+ medium, indicating that the process is reversible (Fig. 4C). The study revealed significant (P < 0.05) interaction between Pi and Suc on the development of lateral roots.
Figure 4.
Effect of Pi and/or Suc availability on lateral root development. Wild-type seedlings grown under different nutrient conditions were documented for the number of primary and higher order laterals after 7 d (A), 14 d (B), and replenishment with P+S+ for 7 d (C). Values are means ± se; n = 20. Different letters indicate that the means differ significantly (P < 0.05).
Interaction between Suc and Auxin in Mediating Pi Deficiency-Induced Modulation of Lateral Root Development
Auxin is essential for lateral root initiation (Bhalerao et al., 2002; Ljung et al., 2005). We therefore examined the effect of Pi and/or Suc deprivation on the distribution of auxin-responsive sites in different parts of the root system of transgenic Arabidopsis expressing DR5∷uidA. Primary and lateral root tips of the seedlings grown under different nutrient conditions showed strong GUS staining, whereas it was barely detectable in the hypocotyl-to-root junction (Fig. 3A). Further, the number of GUS-stained areas was scored in four distinct segments of the root system represented as a, b, c, and d (Fig. 5). GUS expression in the designated areas revealed various developmental stages (I–IV) ranging from primordium initiation to postemergence of the lateral root (Supplemental Fig. S1). Under the P+S+ condition, areas showing GUS expression in region a were significantly higher compared to the other treatments, indicating the potential sites for the development of new laterals. This result was consistent with our earlier observation that clearly showed the ability of the seedling to continuously form new laterals under Pi and Suc sufficiency conditions. On the other hand, there were significantly lower numbers of GUS staining sites in region a and a relatively higher number in region d of P−S+ seedlings. Sporadic GUS-staining areas were also identified in region b, but the differences were not statistically significant (P < 0.05) among the treatments. Although the total number of regions e showing GUS expression in the root system of P+S+ and P−S+ seedlings did not reveal any significant difference (P < 0.05), a shift from primary to lateral roots was distinct during Pi deprivation. Comparatively, Suc deprivation resulted in more drastic reduction in overall e GUS expression in P+S− and P−S− seedlings, highlighting the pivotal role of Suc in making the root system more responsive to auxin.
Figure 5.
Effect of Pi and/or Suc availability on the expression of auxin-responsive reporter DR5∷uidA in the root system. Transgenic DR5∷uidA Arabidopsis seedlings were grown on different nutrient media for 7 d followed by histochemical GUS staining. Schematic illustration of the seedling depicting different regions (a, b, c, and d) on the root system that were scored for the number of areas showing GUS expression. a, Region between the primary root tip and the youngest lateral root. b, Region between the hypocotyl-root junction and the youngest lateral root. c, Primary lateral roots. d, Second-order lateral roots. Data correspond to the average number of areas in the designated regions showing GUS expression in 20 seedlings each for different treatments. Values are means ± se.
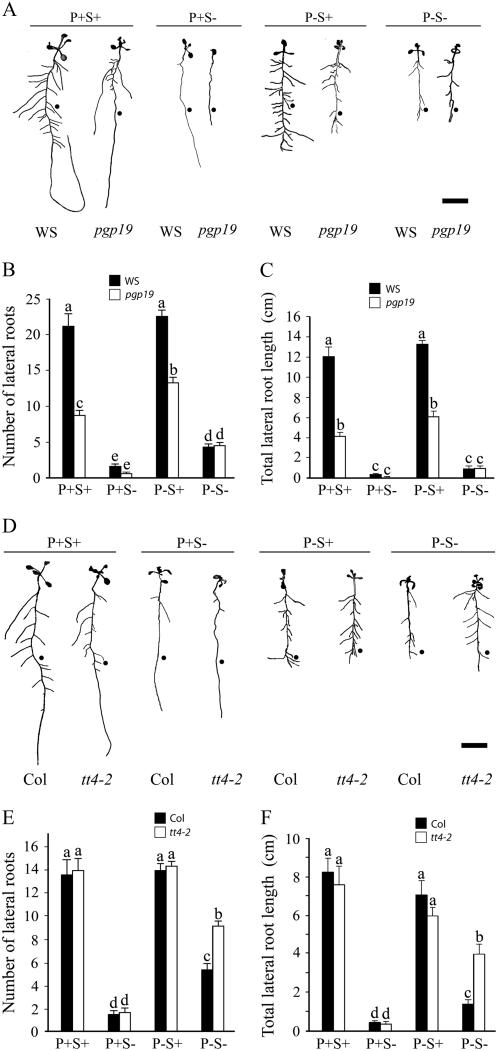
Polar Auxin Transport Mutants Alter Pi Deficiency Responses on Lateral Root Development
We used the pgp19 mutant exhibiting reduced basipetal transport of auxin to and from the root tip (Geisler et al., 2003, 2005) to explore the role of basipetal transport of auxin in Pi deficiency responses (Fig. 6A). The effect of P+S+, P+S−, P−S+, and P−S− conditions on primary root length was comparable in the wild type and pgp19 (data not shown). However, there was significant reduction (P < 0.05) in the number of lateral roots in pgp19 compared to the wild type under P+S+ and P−S+ conditions (Fig. 6B). The effect in pgp19 was less severe under P−S+ conditions (40% reduction) than under P+S+ (60% reduction) conditions. A similar trend was also revealed for the total length of the lateral roots (Fig. 6C). The results indicated Pi deficiency induced increased sensitivity to polarly transported auxin. To investigate further the possible mechanism by which Pi deficiency induces change in root sensitivity to auxin, we evaluated the effects of Pi and/or Suc deprivation on the RSA of the transparent testa mutant tt4-2 (Fig. 6D). Flavonoids have been implicated as natural endoregulators of auxin transport in vivo and absence of flavonoids in transparent testa mutants (tt4-1, tt4-2 tt4-8) results in elevated transport of auxin from the shoot apex to the root tip (Murphy et al., 2000; Brown et al., 2001; Peer et al., 2004). The effect of P+S+, P+S−, P−S+, and P−S− conditions on primary root length were comparable in the wild type and tt4-2 (data not shown). Further, no significant differences (P < 0.05) were observed in the number of lateral roots and total lateral root length in wild-type and mutant seedlings grown under P+S+, P+S−, and P−S+ conditions (Fig. 6, E and F). Comparatively, a significant increase (P < 0.05) in the number of lateral roots under P−S− conditions in the wild type indicated an increase in the sensitivity of the Pi-deprived roots to transported auxin and the effect was significantly accentuated (P < 0.05) in tt4-2 (Fig. 6, E and F). The growth conditions used in our study differed from those of previous studies using tt4 and may also account for the observed differences in the root phenotype. The interaction between Pi and Suc on lateral root development in the wild type and auxin transport mutants (pgp19 and tt4-2) was statistically significant (P < 0.05).
Figure 6.
Auxin transport mutant alters Pi and Suc response on lateral root development. Wild-type and auxin transport mutants pgp19 (A–C) and tt4-2 (D–F) were grown on different nutrient media for 7 d. A and D, Lateral roots were spread to reveal the architectural details. RSAs are representative of 20 seedlings each for different treatments. •, Root length (average = 1.98 cm; n = 80) at the time of transfer to different nutrient media. Scale bar = 1 cm. Data are presented for the number of lateral roots (B and E) and total lateral root length (C and F). Values are mean ± se; n = 20. Different letters indicate that the means differ significantly (P < 0.05).
Pi Deficiency Promotes Suc-Mediated Development of Root Hairs
Increased root hair length and density is also one of the adaptive responses to Pi deficiency (Gahoonia and Nielsen, 1997; Lynch and Brown, 2001; Ma et al., 2001). We therefore evaluated the role of Suc in Pi deficiency-induced development of root hairs in a 5-mm section from the tip of the primary roots of the seedlings grown under different nutrient conditions for 2 d (Fig. 7). Under P+S+ conditions, there was sparse development of root hairs on the primary root (Fig. 7B). The number of root hairs reduced significantly (P < 0.05) in the seedlings grown under P+S− conditions. However, the effect of Suc deprivation was less pronounced on the number of root hairs in P−S− seedlings and was comparable to the P+S+ seedlings, whereas under P−S+ conditions there was about a 3-fold increase in the number of root hairs as compared to P+S+ and P−S− seedlings. Relatively, root hair length was markedly longer under P−S+ conditions than those formed under other nutrient conditions (Fig. 7C). The result clearly illustrated the pivotal role of Suc in Pi deficiency-mediated development of root hairs on the primary root. The interaction between Pi and Suc on the number and length of root hairs was statistically significant (P < 0.05).
Figure 7.
Effect of Pi and Suc availability on root hair development. Development of root hairs in a 5-mm section from the primary root tip of the wild-type seedlings grown on different nutrient media for 2 d. Data are presented for the number of root hairs (B) and root hair length (C). Scale bar = 0.5 mm. Values are means ± se; n = 20. Different letters indicate that the means differ significantly (P < 0.05).
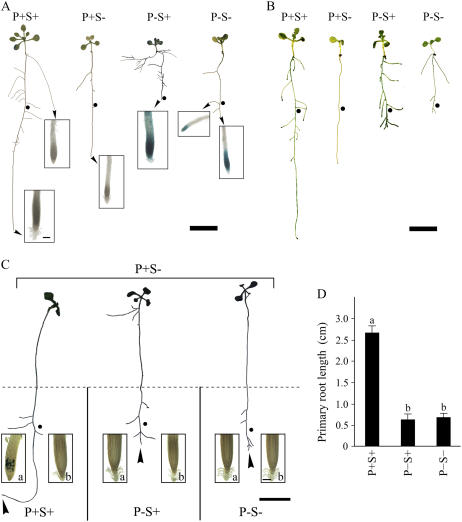
Conditional Cross Talk between Pi Starvation Responses Regulated by Local and Systemic Status of Pi
Cross talk between local and systemic Pi sensing in eliciting Pi starvation responses has been suggested in an earlier study (Sánchez-Calderón et al., 2006). We examined the effect of homogenous and heterogenous Pi supply on the said cross talk in the transgenic Arabidopsis-expressing promoter of the high-affinity Pi-transporter Pht1;4 fused to the GUS reporter (Karthikeyan et al., 2002). Under the homogenous Pi deprivation condition, strong expression of Pht1:4∷uidA in the whole-root system and modulated RSA (Fig. 8A) suggested the prevalence of cross talk between local and systemic responses. On the contrary, under P−S− conditions, despite the inhibition of primary root growth, expression of Pht1;4∷uidA was largely confined to the primary and lateral root tips. Likewise, P+S− conditions reduced primary root growth and the expression of Pht1;4∷uidA was barely detectable in the root system. Because root-associated acid phosphatase (APase) activity increases in Pi-deficient Arabidopsis plants to enhance the release of Pi from organic phosphorus sources (Trull and Deikman, 1998), seedlings grown under different nutrient conditions were stained with 5-bromo-4-chloro-3-indolyl Pi (BCIP) to monitor in vivo activity of APases (Fig. 8B). Although increased APase activity and modulated RSA of P−S+ seedlings suggested possible cross talk between local and systemic responses, there was an apparent lack of such interaction under P−S− conditions. We further used transgenic plants expressing CycB1;1∷uidA and Pht1;4∷uidA in a divided media system to gain further insight into the cross talk in the Pi heterogenous environment (Fig. 8C). Seedlings grown under P+S− conditions for 7 d resulted in plants lacking lateral roots and thus provided ideal growth conditions for monitoring the effect of the heterogenous Pi environment on root traits. P+S− seedlings were transferred to divided agar plates containing P+S− medium in the upper and P+S+, P−S+, or P−S− in the lower section and grown for 7 d. There was a significant increase in primary root length under P+S+ conditions, whereas seedlings with primary root tips growing under P−S+ and P−S− conditions showed significant retardation in their growth (Fig. 8D). The interaction between Pi and Suc was not significant (P < 0.05) on primary root growth. In addition, the distance from the tip to first lateral root was significantly reduced under P−S+ and P−S− conditions as compared to those grown under P+S+ conditions (data not shown) indicative of a typical Pi starvation response despite the upper half of the seedling growing under P+S− conditions. This was further corroborated by the expression of CycB1;1∷uidA in the primary root tip under P+S+ conditions, but complete suppression under P−S+ and P−S− conditions (Fig. 8C, inset a). As was expected, there was no expression of Pht1;4∷uidA under P+S+ conditions. However, expression of Pht1;4∷uidA was suppressed both under P−S+ and P−S− conditions (Fig. 8C, inset b), revealing its systemic repression by the shoot growing under the P+S− region. Together, the results suggested lack of cross talk between local and systemic Pi sensing in eliciting Pi starvation responses. Apparent cross talk observed between these two Pi-sensing mechanisms under P−S+ conditions in the homogenous Pi environment could therefore be a reflection of their independent coexistence.
Figure 8.
Effects of homogenous and heterogenous Pi environment on local and systemic Pi starvation responses. A and B, Transgenic Pht1;4∷uidA Arabidopsis seedlings were grown on different nutrient media for 7 d. RSAs are representative of 20 seedlings each for different treatments. •, Root length (average = 1.8 cm; n = 80) at the time of transfer to different nutrient media. Scale bar = 1 cm. Insets in A show the primary and lateral root tips of the seedlings analyzed for GUS expression of Pht1;4∷uidA. Scale bar in the inset = 100 μm. B, Seedlings stained with BCIP for evaluation of in vivo APase activity. C, Root growth on divided agar petri plates containing P+S− medium in the upper compartment and P+S+, P−S+, or P−S− in the lower compartment. •, Root length (average = 3.7 cm; n = 80) at the time of transfer to divided plates. Scale bar = 1 cm. Inset shows the primary root tips of the seedlings analyzed for GUS expression of CycB1;1∷uidA (a) and Pht1;4∷uidA (b). Scale bar in the inset = 100 μm. D, Increase in primary root length of the seedlings subsequent to their transfer to divided plates. Values are means ± se; n = 20. Different letters indicate that the means differ significantly (P < 0.05).
DISCUSSION
Sensing of Localized Pi Deficiency by Root Is Independent of Suc Availability in the Nutrient Medium
Arabidopsis seedlings grown under P−S+ conditions revealed significant reductions in fresh weight, shoot-to-root ratio, indicative of preferential allocation of photosynthate to root (Nielsen et al., 2001), soluble Pi content, total leaf area, increase in anthocyanin (Fig. 1, A–E), and concomitant higher expression of the high-affinity Pi transporter Pht1;4 (Fig. 2D). The results suggest the effect of Pi deprivation on overall growth and development of Arabidopsis. Although nutrient medium deprived of Suc augmented Pi starvation responses on the fresh weight of the seedling and leaf area (Fig. 1, A and D), the effects on anthocyanin accumulation in the shoot (Fig. 1E) and expression of Pht1;4 and PLDZ2 (Fig. 2D) were markedly decreased. The data were consistent with the studies revealing the pivotal role of sugar in systemic regulation of Pi starvation responses (Liu et al., 2005; Karthikeyan et al., 2007) and regulation of genes coding for anthocyanin biosynthetic enzymes (Solfanelli et al., 2006). The effect of Suc deprivation was also evident on lateral root formation in P+S− and P−S− seedlings (Fig. 2B). Because lateral roots constitute a significant proportion of the root biomass, its reduction resulted in higher shoot-to-root ratios as compared to those grown under Suc-replete conditions (Fig. 1B). This may be reason for higher shoot-to-root ratios in P−S− plants in spite of reduced lateral roots and leaf area. Further, our analysis revealed significantly higher soluble Pi content in the shoot and root of P−S− seedlings as compared to that in P−S+ seedlings (Fig. 1C). This could be attributed to underutilization of the cellular Pi for phosphorylating sugars in Suc-deprived P−S− seedlings (Sadka et al., 1994). The influence of shoot Pi content on the response of primary root growth has been postulated (Williamson et al., 2001). However, our study did not reveal any significant influence of variable levels of Pi in the shoots of P−S+ and P−S− seedlings on the localized Pi deprivation-induced irreversible inhibition of primary root growth (Fig. 2B). Under Pi-sufficient conditions, 3-fold higher shoot Pi content in pho2 as compared to the wild type also did not significantly influence the primary root length (data not shown). Our results are in agreement with the study demonstrating the effect of localized Pi deprivation on root growth to be independent of shoot Pi status (Linkohr et al., 2002). Analysis of a conditional pdr2 mutant that disrupts local Pi sensing also exhibited the inconsequential effect of shoot Pi on primary root growth (Ticconi et al., 2004). Although deprivation of Suc in the nutrient medium also resulted in significant reduction in the primary root growth of P+S− and P−S− seedlings (Fig. 2B, top), replenishment with P+S+ revived the growth only in the former (Fig. 2B,bottom). This study, therefore, suggests that the inhibitory effect of localized Pi deprivation on primary root growth is independent of Suc status in the nutrient medium. This view was further supported by a loss of meristematic activity in the primary roots of P−S+ and P−S− seedlings (Fig. 2C), indicating the onset of the determinate growth phase (Sánchez-Calderón et al., 2005, 2006).
Unlike primary roots, which are embryonic in origin, lateral roots are developed postembryonically from mature, nondividing pericycle cells within the parent root (Scheres et al., 1996). Similar to primary roots, lateral roots also exhibit extensive developmental plasticity and are highly responsive to various nutritional cues, including localized Pi deprivation (Linkohr et al., 2002). Our study on the expression analysis of CycB1;1∷uidA in transgenic seedlings grown under P−S+ and P−S− conditions for 7 d revealed inhibition of mitotic activity in the primary roots, whereas it was still maintained in the lateral roots (data not shown) and is in agreement with an earlier study (Sánchez-Calderón et al., 2005). This differential temporal response of primary and lateral roots to localized Pi deprivation could possibly be related to their distinct ontogeny. Upon prolonged Pi starvation for 14 d, primary lateral roots entered a determinate growth phase and triggered the formation of indeterminate higher order lateral roots both under Suc-replete (P−S+) and Suc-deprived (P−S−) conditions (data not shown). Our study thus suggests the ability of both primary and lateral roots to sense localized Pi deprivation regardless of Suc status in the nutrient medium.
The genetic components involved in the root growth responses to local Pi availability are now beginning to be unraveled (Ticconi et al., 2004; Reymond et al., 2006; Sánchez-Calderón et al., 2006). Molecular evidence has also been provided by morphological analysis of the pldz2 mutant, revealing early loss of the meristem structure in the primary root (Cruz-Ramírez et al., 2006). Our result showed a significant increase in the expression of PLDZ2 in the roots of P−S+ seedlings as compared to that in P+S+ and P+S− seedlings (Fig. 2D) and this observation was in agreement with the induction of PLDZ2 during Pi deprivation (Misson et al., 2005). However, relative to P−S+ conditions, there was a significant reduction in the transcripts of both PLDZ2 and the high-affinity Pi transporter Pht1;4 under P−S− conditions. This pattern of expression was consistent with the hypothesis that sugar plays a pivotal role in the regulation of PSI genes (Liu et al., 2005; Karthikeyan et al., 2007). Because both P−S+ and P−S− conditions induced determinate root growth (Fig. 2B), the differential response of PLDZ2 to Suc suggested likely involvement of other molecular determinants as well that may regulate the Pi starvation-mediated meristematic activity of the primary root in a sugar-independent manner. Some evidence has been provided for the notion that Pi deficiency-induced inhibition of cell division in the root meristem is preceded by developmental changes in the quiescent center (Sánchez-Calderón et al., 2005). However, presently it is not known whether Suc plays any role in the quiescent center response to Pi deprivation and this certainly merits further investigation. Efforts are now under way to analyze global spatiotemporal gene expression during Pi deficiency both under Suc-replete and Suc-deprived conditions using the Affymetrix ATH1 GeneChip. We have used this technology in our earlier study for global analysis of PSI genes (Mission et al., 2005).
Auxin Does Not Affect Pi Deficiency-Induced Determinate Primary Root Growth
Because auxin has been implicated in root growth and its adaptive responses to different environmental cues (Reed et al., 1998; Malamy and Ryan, 2001), several studies investigated its role in Pi deficiency-induced reduction of primary root growth (Williamson et al., 2001; López-Bucio et al., 2002, 2005; Al-Ghazi et al., 2003; Nacry et al., 2005). Interestingly, auxin-resistant mutants axr1, axr2, and axr4 revealed wild-type responses both under Pi-sufficient and Pi-deficient conditions, pointing toward the auxin-independent response of Pi deficiency on primary root growth (Williamson et al., 2001; López-Bucio et al., 2002; Al-Ghazi et al., 2003). Consistent with this hypothesis, our analysis also showed comparable effects of Pi and/or Suc deprivation on primary root growth of the wild-type and chalcone synthase mutant tt4-2 (Fig. 6D). Although auxin supplementation inhibited primary root growth (Fig. 3D) and mimicked the phenotype of Pi-deprived seedlings (Casimiro et al., 2001; Al-Ghazi et al., 2003; Nacry et al., 2005), it failed to trigger a loss of meristematic activity under Pi-replete conditions (Fig. 3, E and F). There was a lack of correlation between the primary root responses to different nutrient treatments (Fig. 2B, top) and the expression of the auxin-responsive reporter DR5∷uidA and auxin content in the primary root tip (Fig. 3, A and B). Therefore, our study suggests that reduced primary root growth either due to auxin supplementation or by sugar deprivation as observed in P+S− seedlings (Fig. 2B, top) does not necessarily reflect a loss of meristematic activity in the primary root, which is a rather specific response to Pi deprivation. A similar inference was drawn by demonstrating the inability of auxin to either mimic or rescue the root phenotype of the pdr2 mutant showing disruption in the local Pi-sensing mechanism (Ticconi et al., 2004). Therefore, modulation of the mitotic activity of the primary root by the Pi-specific local sensing pathway lying downstream of the auxin-mediated maintenance of the root meristem could be postulated (Ticconi et al., 2004; Jiang and Feldman, 2005).
Cross Talk between Suc and Auxin on the Pi Deficiency Response on Lateral Roots
The availability of nutrients influences both the number and location of lateral root initiation sites (Drew and Saker, 1978). Pi deficiency-induced modulation in lateral root development reflects adaptive responses to develop a shallow root system for enhanced foraging of the surface soil horizons where Pi availability is relatively high, particularly in stratified soils with heterogenous phosphorus distribution (Ge et al., 2000). In this study, we show severe inhibition of lateral roots in seedlings grown under P+S− conditions (Figs. 2B and 6, A and D). The phenotype mimicked the ones obtained by either manipulating Suc or nitrogen in the medium (Malamy and Ryan, 2001) or localized application of the auxin transport inhibitor naphthylphthalamic acid at the root-to-shoot junction (Reed et al., 1998). RSA analysis revealed the pivotal role of Suc in the development of lateral roots and is in agreement with the proposed role of sugar in mediating some RSA responses to environmental cues (Freixes et al., 2002). Furthermore, our study suggests potential involvement of Suc in acropetal transport of auxin from shoot to root, which is pivotal for lateral root formation (Casimiro et al., 2001). However, Suc deprivation did not influence the basipetal auxin transport-mediated gravitropic response of P+S− seedlings (data not shown). Earlier study had also shown normal gravitropic responses of the excised root tips (Rashotte et al., 2000). Because basipetal transport of auxin in root apical tissues also mediates an initiation phase of lateral root development (Bhalerao et al., 2002), Suc-deprived Pi-deficient medium (P−S−) provided ideal conditions to assess the role of auxin in eliciting a Pi deficiency response on lateral root development. Under P−S− conditions, initiation of a few lateral roots (Figs. 2B and 6, A and D) correlated with elevated root basipetal transport, particularly in the most apical 2 mm of its root tip (Fig. 3C). This increase in transport may have resulted from increased pyrophosphate-dependent apoplastic acidification (Li et al., 2005), which would have activated under Suc- and Pi-deficient conditions. Further, RSA analysis of pgp19 grown under P−S+ conditions revealed significant reduction in the number of lateral roots as compared to the wild type (Fig. 6B). Together, these results suggest Pi deficiency induced increased sensitivity of the roots to basipetally transported auxin.
Relative to P−S− seedlings, P−S+ seedlings exhibited significantly higher initiation of lateral roots (Figs. 2B and 6, A and D), which could be an integrated response to both shoot-derived and elevated basipetal transport of auxin. In addition, our data show a shift in auxin responsiveness from primary roots in P+S+ seedlings to lateral roots in P−S+ seedlings (Fig. 5), which suggests a redistribution of auxin during Pi deprivation and is in agreement with an earlier study (Nacry et al., 2005). The lateral root primordia also become competent to synthesize auxin 3 to 10 d after germination (Ljung et al., 2005). This was evident from our results as well showing, regardless of Pi and/or sugar availability, consistently strong expression of DR5∷uidA in the tips of primary and lateral roots (Fig. 3A). Our results and those of other studies, therefore, suggest differential auxin fluxes from multiple sources in mediating Pi starvation responses on RSA.
The mechanism involved in Pi deficiency-induced changes in local auxin concentration or its transport in the root system is still unknown (López-Bucio et al., 2005). Potential candidates influencing this process could be aglycone flavonols, the endogenous regulators of polar auxin transport (Jacobs and Rubery, 1988). Earlier studies had shown that auxin transport is enhanced in the absence of flavonoids (Murphy et al., 2000; Brown et al., 2001). Under P−S− conditions, RSA analysis of the chalcone synthase mutant tt4-2 revealed a significantly higher number and longer lateral roots compared to the wild type (Fig. 6, E and F). The study suggests that the effect on auxin transport or signaling in the flavonoid-deficient mutant is Pi dependent. Our earlier study had also shown that genes involved in flavonoid biosynthesis are induced 2- to 4-fold during Pi starvation (Misson et al., 2005). However, more detailed studies are warranted to draw any conclusive evidence on the effect of Pi deprivation on the spatiotemporal distribution of different flavonoids and their role in exerting influence on auxin-mediated alteration of RSA.
In conclusion, we provide evidence for the differential roles of sugar and auxin in mediating Pi deficiency-induced modulation of primary and lateral root development. Furthermore, increased root hair formation during Pi deprivation has been attributed to the ectopic development of hairs in the N position normally occupied by nonhair cells (Muller and Schmidt, 2004). Consistent with these reports, our results also showed Suc-dependent Pi deficiency induced higher root hair density on primary roots of P−S+ seedlings (Fig. 7). The complexity and multitude of effects of Pi deficiency could be further gauged from the fact that there is an apparent lack of cross talk between localized and systemic starvation responses (Fig. 8). In addition, other signaling pathways involving cytokinins and ethylene also exert their influences on molecular and RSA responses to Pi deprivation (Karthikeyan et al., 2002; Ma et al., 2003; Franco-Zorrilla et al., 2005). However, it still remains to be deciphered how different signaling pathways interact to elicit their effects on localized and systemic Pi sensing that integrate Pi starvation responses.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotypes Columbia (Col-0) and Wassilewskija, mutants pgp19 in the Wassilewskija background (Geisler et al., 2005), tt4-2 in the Col-0 background (Bennett et al., 2006), pho2 in the Col-0 background (Delhaize and Randall, 1995), and seeds of transgenic CycB1;1∷uidA (Colón-Carmona et al., 1999) and DR5∷uidA (Ulmasov et al., 1997) were used in this study. Seeds were surface sterilized by treating with 70% (v/v) ethanol for a minute and rinsed twice with sterile water. The seeds were then treated with a solution containing 50% (v/v) commercial bleach and 0.1% (v/v) Tween20 for 10 min, followed by several washes with sterile water to remove the traces of bleach. The surface-sterilized seeds were suspended in sterile water and stratified for 2 d at 4°C and subsequently suspended in 0.14% (w/v) agar. Plating density was roughly between 25 to 30 seeds per petri plate. Plates were placed vertically under controlled conditions in tissue culture room (16-h day/8-h night cycle at 22°C and average photosynthetically active radiation [PAR] of 25 to 30 μmol m−2 s−1 provided by florescent tubes). Low PAR conditions were used to minimize the effects of photosynthates produced by the seedlings on the treatments. In our previous studies, we had used low PAR conditions that showed normal responses on the expression of P+-induced light-regulated psbO and psbP genes (Jain et al., 2005) and Pi deficiency-induced high-affinity Pi transporters (Karthikeyan et al., 2002, 2007). For all the experiments, seeds were grown initially on one-half-strength Murashige and Skoog medium, containing 1.2% (w/v) agar and 1.5% (w/v) Suc in petri plates (150 × 15 mm) for 5 d. Uniformly grown seedlings with primary root length in the range of 1.5 to 2.5 cm were selected to minimize the effect of intrinsic variability on the subsequent treatments of P+S+, P+S−, P−S+, and P−S−. P+ constituted high phosphorus (1.25 mm KH2PO4) in modified Murashige and Skoog medium (López-Bucio et al., 2002), pH 5.7, and 1.2% (w/v) agar. KH2PO4 in Murashige and Skoog medium was replaced with K2SO4 for P− medium. Agar (A-1296; Sigma) used in this study was analyzed for elemental composition by inductively coupled plasma-mass spectrometry, which revealed an impurity of 20 μm total Pi (J. Ward and K.G. Raghothama, unpublished data). Therefore, in this study, P− refers to 20 μm Pi. S+ and S− represented 1.5% Suc and Suc-deprived medium, respectively. For each of the treatments, 20 selected seedlings were transferred (five per plate) and grown for 7 d for all the experiments, unless stated otherwise. For root growth study in a heterogenous Pi environment, transgenic Pht1;4∷uidA and cycB1∷uidA seedlings were grown on one-half-strength Murashige and Skoog + 1.5% Suc for 5 d on vertically oriented petri plates and subsequently grown for another 7 d on P+S− conditions before transferring them to divided agar petri plates containing P+S− medium in the upper compartment and P+S+, P−S+, or P−S− in the lower compartment. Primary root axis of each seedling was aligned to ensure the placement of about 2.5 cm and 1.0 to 1.5 cm in the upper and lower compartments, respectively. Root-bending assay was performed to determine the effect of treatments on the gravitropic response of the seedlings. Seedlings grown on one-half-strength Murashige and Skoog + 1.5% Suc for 5 d on vertically oriented petri plates were transferred to square petri plates and placed vertically to facilitate root growth downward for 7 d. For auxin supplementation experiments, IAA (0.1 μm) was added to the cooled (50°C) molten medium from a stock dissolved in absolute ethanol with a final ethanol concentration of 0.01%.
Root Hairs
Seedlings were grown on one-half-strength Murashige and Skoog medium containing 1.2% (w/v) agar and 1.5% (w/v) Suc in petri plates for 5 d and then transferred to agar plates containing different nutrient media. Two days after transfer, images of the root hairs growing in the 5-mm section from the tip of the primary root were captured using a stereomicroscope (Nikon SMZ-U). The ImageJ (a Java image-processing program; http://rsb.info.nih.gov/ij) program was used for analyzing the captured images for number and length of the root hairs.
Analysis of RSA
Seedlings were scanned at 600 dpi directly from the petri plate by using a desktop scanner (UMAX Powelook 2100 XL). Scanned images were used for measuring an increase in primary root length after being transferred from one-half-strength Murashige and Skoog + 1.5% Suc to different nutrient media by using ImageJ. The number of primary and higher order laterals were documented under stereomicroscopy by gently spreading the lateral roots in a pool of water.
Analysis of Morphological Traits
Under stereomicroscopy, leaves were separated from the seedlings, transferred to agar plates to keep them moist, scanned using a desktop scanner, and total leaf area of individual leaves was measured by employing ImageJ. For shoot-to-root fresh weight biomass ratio, roots were separated from the seedling at the root-hypocotyl junction.
Quantification of Anthocyanin and Soluble Pi Content
Shoot tissue was used for the quantification of anthocyanins as described in Lange et al. (1971). The optical density was measured at A532 and A653. Subtraction of 0.24 A653 compensated for the small overlap in A532 by the chlorophylls (Murray and Hackett, 1991). The concentration was determined by using the corrected absorbance and the molar extinction coefficient (ɛ) of 38,000 L mol−1 cm−1 for anthocyanin (Hrazdina et al., 1982). For estimation of soluble Pi content, shoot and roots were separated, rinsed in distilled water, blotted dry, frozen, and ground to a fine powder in liquid nitrogen. About 20 to 25 mg of the ground tissue was suspended in 250 μL of 1% glacial acetic acid, vortexed, and centrifuged to pellet the cellular debris. The supernatant was assayed for Pi using a phosphomolybdate colorimetric assay according to Ames (1966).
APase Analysis
For visualization of in vivo APase activity, seedlings grown under different nutrient conditions for 7 d were layered gently with 0.6% agar containing 0.04% BCIP in 50 mm sodium acetate (pH 4.9) and 10 mm MgCl2 (Trull and Deikman, 1998) and transferred back to normal growth conditions for overnight staining. Seedlings were scanned at 600 dpi directly from the petri plate by using a desktop scanner (UMAX Powelook 2100 XL).
Histochemical Analysis
For histochemical analysis of the GUS reporter enzyme activity in Arabidopsis transgenic lines, DR5∷uidA and Pht1;4∷uidA, seedlings were fixed in 4% (w/v) paraformaldehyde, 0.5% (v/v) glutaraldehyde in 0.1 m Pi buffer for 1 to 2 min, rinsed twice with 0.1 Pi buffer, and incubated for 12 h in reaction buffer containing 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid as the substrate (Craig, 1992). For histochemical analysis of GUS activity in Arabidopsis transgenic line CycB1:1, GUS reaction buffer was supplemented with 5 mm each of K3Fe(CN)6 and K4Fe(CN)6 3H2O (Malamy and Ryan 2001). Stained seedlings were cleared with ethanol and at least 10 to 15 samples for each of the treatments for different marker lines were analyzed for GUS-staining patterns under compound microscopy.
Free IAA Determination and Auxin Transport Assay
Seedlings grown under different nutrient conditions for 7 d were harvested, cotyledons excised at the root-shoot junction, and free IAA quantified in lower (4 mm) and upper roots as described in Geisler et al. (2005). For polar transport of [3H] IAA assay, seedlings were grown initially on one-half-strength Murashige and Skoog + 1.5% Suc for 3 d and then transferred to different nutrient conditions for 3 d; then root segments 2, 4, and 6 mm from the root tip were excised for analysis as described in Geisler et al. (2005).
Real-Time PCR
Total RNA was isolated from roots of the seedlings grown under different nutrient conditions for 7 d by using the Qiagen RNeasy plant mini kit (Qiagen). RNA was treated with RQ1 RNase-free DNase I (Promega) according to the manufacturer's instructions. Three micrograms of total RNA were reverse transcribed with the SuperScript III first-strand synthesis kit (Invitrogen). Real-time PCR was performed with an Applied Biosystems 7500 real-time PCR system using SYBR Green detection chemistry (Applied Biosystems) and gene-specific primers. 18S ribosomal RNA was used as an internal control and the relative expression levels of the genes was computed by the 2−ΔΔCt method of relative quantification (Livak and Schmittgen, 2001). The expression value for Pht1;4 and PLDZ2 in the P+S+ sample was set at 1.
Statistical Analysis
Data were analyzed by ANOVA using the SPSS 10 program. Univariate and multivariate analytical procedures with a Tukey's honestly significant difference mean-separation test were used for evaluating the interaction between Pi and Suc supply to different genotypes. Different letters on the histograms indicate means that were statistically different at P ≤ 0.05.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Histochemical GUS staining of transgenic DR5∷uidA Arabidopsis seedlings showing different stages of lateral root development ranging from primordia initiation (stage I) to postemergence (stage IV).
Supplementary Material
Acknowledgments
We thank Dr. Paul M. Hasegawa, Purdue University, for his useful suggestions. We also thank Dr. Jocelyn E. Malamy (University of Chicago), for her valuable insights on lateral root development in Arabidopsis, Dr. Luis Herrera-Estrella (Laboratorio Nacional de Genómica para la Biodiversidad, México), and Dr. Hisashi Koiwa (Texas A&M University) for the seeds of CycB1:1∷uidA transgenic plants. We are also grateful to Debra M. Sherman and Chia-Ping Huang (Purdue University) and Aaron P. Smith (University of Georgia) for their help in microscopy work and real-time PCR analysis, respectively.
This work was supported by the U.S. Department of Agriculture and McKnight grants (to K.G.R.) and the U.S. Department of Agriculture-National Research Initiative (grant to A.S.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kashchandra G. Raghothama (kragu@purdue.edu).
Some figures in this article are displayed in color online but in black and white in print.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P (2003) Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signaling. Plant Cell Environ 26 1053–1066 [Google Scholar]
- Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8 115–118 [Google Scholar]
- Baldwin JC, Karthikeyan AS, Raghothama KG (2001) LEPS2, a phosphorus starvation-induced novel acid phosphatase from tomato. Plant Physiol 125 728–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16 553–563 [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29 325–332 [DOI] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh SH, Harrison MJ (1999) The down regulation of Mt4-like genes by phosphate fertilization occurs systemically and involves phosphate translocation to the shoots. Plant Physiol 119 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inze D, Sandberg G, Casero PJ, et al (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F, Pata M, Nacry P, Doumas P, Rossignol M (2003) Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant Cell Environ 26 1839–1850 [Google Scholar]
- Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P (1999) Spatio temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20 503–508 [DOI] [PubMed] [Google Scholar]
- Craig S (1992) The GUS reporter gene. Application to light and transmission electron microscopy. In SR Gallagher, ed, GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression. Academic Press, San Diego, pp 115–124
- Cruz-Ramírez A, Oropeza-Aburto A, Razo-Hernández F, Ramírez-Chávez E, Herrera-Estrella L (2006) Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc Natl Acad Sci USA 103 6765–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Randall PJ (1995) Characterization of a phosphate accumulator mutant of Arabidopsis thaliana. Genes Dev 9 2131–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, Saker LR (1978) Nutrient supply and the growth of the seminal root system in barley. J Exp Bot 29 435–451 [Google Scholar]
- Ferreira PC, Hemerly AS, Engler JD, van Montagu M, Engler G, Inze D (1994) Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6 1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG (2002) The role of long-distance signaling in plant responses to nitrate and other nutrients. J Exp Bot 53 39–43 [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Martín AC, Leyva A, Paz-Ares J (2005) Interaction between phosphate starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol 138 847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freixes S, Thibaut M-C, Tardieu F, Muller B (2002) Root elongation and branching is related to local hexose concentration in Arabidopsis thaliana seedlings. Plant Cell Environ 25 1357–1366 [Google Scholar]
- Gahoonia TS, Nielsen NE (1997) Variation in root hairs of barley cultivars doubled soil phosphorus uptake. Euphytica 98 177–182 [Google Scholar]
- Ge Z, Rubio G, Lynch J (2000) The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model. Plant Soil 218 159–171 [DOI] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwantanakun B, Peer WA, Bailly A, Richards EL, et al (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44 179–194 [DOI] [PubMed] [Google Scholar]
- Geisler M, Kolukisaoglu HU, Bouchard R, Billion K, Berger J, Saal B, Frangne N, Koncz-Kálmán Z, Koncz C, Dudler R, et al (2003) TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol Biol Cell 14 4238–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL (2000) Proteoid root development of phosphorus-deficient lupin is mimicked by auxin and phosphate. Ann Bot (Lond) 85 921–928 [Google Scholar]
- Hrazdina G, Marx GA, Hoch HC (1982) Distribution of secondary plant metabolites and their biosynthetic enzymes in pea (Pisum sativum L.) leaves: anthocyanins and flavonol glycosides. Plant Physiol 70 745–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH (1988) Naturally occurring auxin transport regulators. Science 241 346–349 [DOI] [PubMed] [Google Scholar]
- Jain A, Cao A, Karthikeyan AS, Baldwin JC, Raghothama KG (2005) Phosphate deficiency suppresses the expression of light-regulated psbO and psbP genes encoding extrinsic proteins of oxygen-evolving complex of PSII. Curr Sci 88 1592–1596 [Google Scholar]
- Jiang K, Feldman LJ (2005) Regulation of root apical meristem development. Annu Rev Cell Dev Biol 21 485–509 [DOI] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG (2007) Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta 225 907–918 [DOI] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, Raghothama KG (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange H, Shropshire W Jr, Mohr H (1971) An analysis of phytochrome-mediated anthocyanin synthesis. Plant Physiol 47 649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, Titapiwantakun B, Undurraga S, Khodakovskaya M, Richards EL, et al (2005) Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 310 121–125 [DOI] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HMO (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J 29 751–760 [DOI] [PubMed] [Google Scholar]
- Liu J, Samac DA, Bucciarelli B, Allan DL, Carroll P, Vance CP (2005) Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. Plant J 41 257–268 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Nonmanly J, Sandberg G (2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17 1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Sompson J, Herrera-Estrella L (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Pérez-Torres A, Rampey RA, Bartel B, Herrera-Estrella L (2005) An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis: identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol 137 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP (1995) Root architecture and plant productivity. Plant Physiol 109 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Brown KM (2001) Topsoil foraging: an architectural adaptation of plants to low phosphorus availability. Plant Soil 237 225–237 [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ 29 459–467 [Google Scholar]
- Malamy JE, Ryan KS (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127 899–909 [PMC free article] [PubMed] [Google Scholar]
- Martín AC, del Pozo JC, Iglesias J, Rubio V, Solano R, de la Peña A, Leyva A, Paz-Ares J (2000) Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. Plant J 24 559–567 [DOI] [PubMed] [Google Scholar]
- Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, Bligny R, Ortet P, Creff A, Somerville S, Rolland N, et al (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon JF, Daynard TB, Van Duinen B, Tollenaar M (1984) Comparison among rates of appearance of leaf tios, collars and leaf area in maize (Zea mays L.). Maydica 29 109–120 [Google Scholar]
- Muller M, Schmidt W (2004) Environmentally induced plasticity of root hair development in Arabidopsis. Plant Physiol 134 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A, Peer W, Taiz L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211 315–324 [DOI] [PubMed] [Google Scholar]
- Murray JR, Hackett WP (1991) Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedra helix L. Plant Physiol 97 343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, Azmi A, Onckelen HV, Rossignol M, Doumas P (2005) A role for auxin redistribution in the response of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol 138 2061–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KL, Eshel A, Lynch JP (2001) The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J Exp Bot 52 329–339 [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274 37–49 [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday G (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond M, Svistoonoff S, Loudet O, Nussaume L, Desnos T (2006) Identification of QTL controlling root growth response to phosphorus starvation in Arabidopsis thaliana. Plant Cell Environ 29 115–125 [DOI] [PubMed] [Google Scholar]
- Rolland F, Sheen J (2005) Sugar sensing and signalling networks in plants. Biochem Soc Trans 33 269–271 [DOI] [PubMed] [Google Scholar]
- Sadka A, DeWald DB, May GD, Park WD, Mullet JE (1994) Phosphate modulates transcription of soybean VspB and other sugar-inducible genes. Plant Cell 6 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46 174–184 [DOI] [PubMed] [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Gutiérrez-Ortega A, Hernández-Abreu E, Herrera-Estrella L (2006) Characterization of low phosphorus insensitive mutants reveals a cross talk between low phosphorus-induced determinate root development and the activation of genes involved in the adaptation of Arabidopsis to phosphorus deficiency. Plant Physiol 140 879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B, McKhann HI, van den Berg C (1996) Roots redefined: anatomical and genetic analysis of root development. Plant Physiol 111 959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S (2004) Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J 37 801–814 [DOI] [PubMed] [Google Scholar]
- Trull MC, Deikman J (1998) An Arabidopsis mutant missing one acid phosphatase isoform. Planta 206 544–550 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157 423–447 [DOI] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux S, Fitter AH, Leyser O (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126 875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Forde B (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279 407–409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.