Abstract
BOB.1/OBF.1 is a transcriptional coactivator essential at several stages of B-cell development. In T cells, BOB.1/OBF.1 expression is inducible by co-stimulation. However, a defined role of BOB.1/OBF.1 for T-cell function had not been discovered so far. Here, we show that BOB.1/OBF.1 is critical for T helper cell function. BOB.1/OBF.1−/− mice showed imbalanced immune responses, resulting in increased susceptibility to Leishmania major infection. Functional analyses revealed specific defects in TH1 and TH2 cells. Whereas expression levels of TH1 cytokines were reduced, the secretion of TH2 cytokines was increased. BOB.1/OBF.1 directly contributes to the IFNγ and IL2 promoter activities. In contrast, increased TH2 cytokine production is controlled indirectly, probably via the transcription factor PU.1, the expression of which is regulated by BOB.1/OBF.1. Thus, BOB.1/OBF.1 regulates the balance of TH1 versus TH2 mediated immunity.
Keywords: BOB.1/OBF.1, IFNγ, Leishmania major, PU.1, T helper cells
Introduction
T helper cells are classified according to their production of defined cytokines. TH1 cells secrete high amounts of IFNγ and IL2, thereby inducing cell-mediated immunity, mainly through the activation of macrophages. TH2 cells secrete IL4, IL5 and IL13, thereby affecting the humoral immune responses (Liew, 2002). Whether a naïve CD4T cell develops into the TH1 or TH2 lineage primarily depends on the signals the cells receive during presentation of the antigen by the APC, as well as on the type of antigen (Murphy et al, 2000).
Activation or silencing of specific genes in TH1 or TH2 lineages is controlled by complex mechanisms including transcriptional regulation and epigenetic modifications (Murphy and Reiner, 2002; Ansel et al, 2003). The IL4-induced STAT6 activation leads to the rapid expression of the transcription factor GATA-3, the master regulator of TH2 lineage development. In contrast, the transcription factor T-bet was identified as an important regulator of TH1 development. T-bet is expressed upon IFNγ-induced STAT1 activation and promotes induction of IFNγ expression in TH1 cells. In addition to T-bet and STATs (Soutto et al, 2002; Tong et al, 2005), several transcription factors were shown to regulate IFNγ promoter activity, like NF-AT (Yang et al, 1999), AP-1, CREB, YY1 and NF-κB (Cippitelli et al, 1995; Ye et al, 1996; Sica et al, 1997; Sweetser et al, 1998; Zhang et al, 1998). However, IFNγ gene regulation is not yet completely understood.
Several studies described the importance of an efficient TH1 response for host immunity against the protozoan parasite Leishmania major (Sacks and Noben-Trauth, 2002). In the murine model, the control of infection with a high dose of L. major in genetically resistant strains, such as CBA/J, C57BL/6 and C3H, ultimately depends on the IL12-driven release of TH1-derived IFNγ. This activates infected macrophages to eliminate parasites and thus leads to containment of the parasites. In contrast, infection of susceptible Balb/c mice leads to progressive, non-healing lesions associated with an early and dominant TH2 response (Sacks and Noben-Trauth, 2002).
BOB.1/OBF.1 (also named OCA-B) was originally described as a B-cell-specific transcriptional coactivator (Luo et al, 1992; Gstaiger et al, 1995; Pfisterer et al, 1995; Strubin et al, 1995). When recruited to DNA via protein–protein interaction with Oct1 or Oct2 it enhances octamer-dependent transcription. BOB.1/OBF.1 is important at multiple stages of B-cell development, in the bone marrow (Schubart et al, 2000; Hess et al, 2001; Brunner et al, 2003b; Siegel et al, 2006), as well as at late stages in secondary lymphoid organs (Nielsen et al, 1996; Hess et al, 2001; Samardzic et al, 2002). BOB.1/OBF.1−/− mice completely fail to form germinal centers upon immunization with thymic-dependent antigens. Consequently, the production of secondary immunoglobulin (Ig) isotypes is massively reduced (Kim et al, 1996; Nielsen et al, 1996; Schubart et al, 1996). Beside the role of BOB.1/OBF.1 as a transcriptional coactivator, a non-transcriptional role was identified. In the cytoplasm, an isoform of BOB.1/OBF.1 is myristoylated and localizes to the membrane (Yu et al, 2001). Cytoplasmic BOB.1/OBF.1 interacts with the Src-kinase Syk and directly regulates Syk stability (Siegel et al, 2006).
In T cells, BOB.1/OBF.1 expression can be induced by treatment with αCD3+αCD28 antibodies, or by co-stimulation with phorbol ester and ionomycin (Sauter and Matthias, 1997; Zwilling et al, 1997; Moriuchi and Moriuchi, 2001). The transactivation function of BOB.1/OBF.1 is also regulated by co-stimulation (Zwilling et al, 1997). BOB.1/OBF.1 expression was also observed in human T-cell lymphomas (Marafioti et al, 2003). Recently it was shown that Lck promoter-driven expression of BOB.1/OBF.1 in mice leads to increased expression of the transcription factor Spi-B in thymocytes, due to the direct activation of an octamer containing Spi-B promoter (P2) by BOB.1/OBF.1 (Bartholdy et al, 2006).
Whereas the role of BOB.1/OBF.1 in B-cell development and function is well documented, the role in T cells remains to be elucidated. Here, we analyzed T-cell development and function in BOB.1/OBF.1−/− mice. We show that BOB.1/OBF.1 is expressed in TH1 as well as in TH2 cells. The cytokine secretion of both subtypes of T helper cells was affected by the BOB.1/OBF.1 deficiency. As a consequence, the resistance in experimental leishmaniasis was severely impaired. We have elucidated the molecular network by which BOB.1/OBF.1 regulates the balance of TH1 versus TH2 activity.
Results
Reduced T-cell numbers in BOB.1/OBF.1-deficient mice
In order to elucidate the function of BOB.1/OBF.1 in T cells, we carefully reanalyzed the T-cell compartment of BOB.1/OBF.1−/− mice. Whereas the cellularity of the thymus was normal and development of thymocytes up to single-positive stages was unaffected (data not shown), splenic T-cell numbers were reduced about two-fold. T-cell subpopulations were also reduced in lymph nodes of BOB.1/OBF.1-deficient animals, albeit less significantly (Figure 1A and B). Both, CD4+ and CD8+ subpopulations were affected. Therefore, the ratio of CD4+ to CD8+ subpopulations in lymph nodes and spleen is unaltered (Supplementary Figure S1). The strong reduction of peripheral B cells had been described previously.
Figure 1.
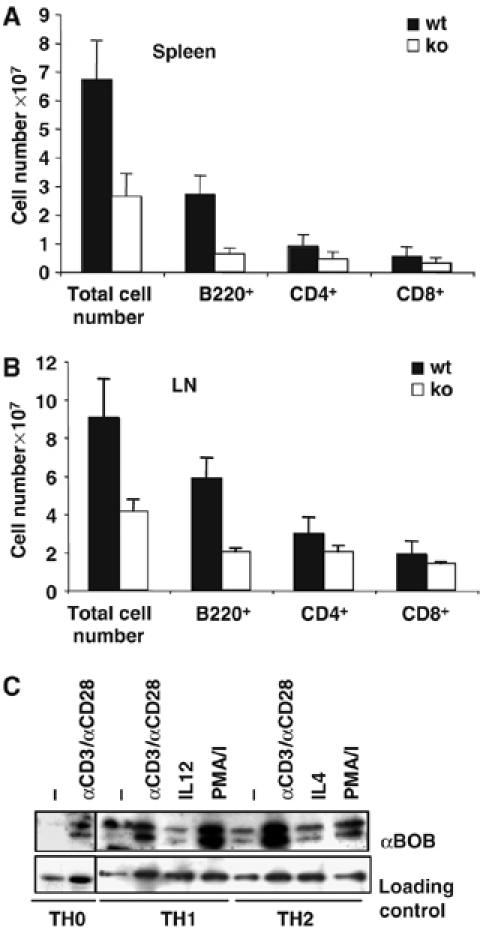
(A, B) T-cell numbers are reduced in BOB.1/OBF.1-deficient mice. (A) Cell suspensions of spleens from wild-type (n=9) and BOB.1/OBF.1−/− mice (n=11) derived from four individual experiments were stained for B220, CD4 or CD8. (B) Analyses of cell suspensions from lymph nodes for B220+, CD4+ and CD8+ cell numbers. Data presented are the mean values (±s.d.) of cell numbers positive for the analyzed cell surface markers. (C) BOB.1/OBF.1 expression in TH0, TH1 and TH2 cells increases after TCR stimulation. Lysates of the indicated cell lineages after 6 days of polarization, re-stimulated with plate-bound αCD3+αCD28, with PMA/ionomycin (PMA/I), IL12 or IL4 or left untreated for 24 h, were resolved by SDS–PAGE and immunoblotted with αBOB.1/OBF.1. For a loading control, the blot was reprobed with αtubulin or αPLCγ1.
To analyze the expression level of BOB.1/OBF.1 in T helper subtypes, immunoblots were performed. Similar to unpolarized CD4+ T cells, BOB.1/OBF.1 is induced in both TH1 and TH2 cells by co-stimulation (αCD3+αCD28), or by treatment with PMA+ionomycin. Stimulation of differentiated T helper cells with IL12 or IL4 alone had no influence on BOB.1/OBF.1 expression (Figure 1C). In contrast to unpolarized CD4+ T cells, which do not show detectable BOB.1/OBF.1 expression without stimulation, polarized TH1 and TH2 cells express low levels of BOB.1/OBF.1. Expression levels can be further increased by re-stimulation of the polarized cells. The polarization efficiency was controlled in parallel by ELISA. This analysis revealed that TH1-polarized cells did not produce TH2 cytokines and vice versa (Supplementary Figure S2). Stimulation with αCD3-specific antibodies alone is sufficient to induce BOB.1/OBF.1 expression, both in naïve T cells and polarized TH1 or TH2 cells (Supplementary Figure S3, data not shown).
BOB.1/OBF.1-deficient mice are not able to control L. major infection
Since BOB.1/OBF.1 is expressed in both main T helper subpopulations, we wondered whether the BOB.1/OBF.1 deficiency leads to physiological consequences for T helper cell function. The infection of mice with L. major is an established and sensitive method to measure the correct TH1/TH2 balance in vivo. The control of L. major infection in genetically resistant strains depends on the consecutive activation of dendritic cells (DCs), followed by an efficient IFNγ -dominated TH1 response. When BOB.1/OBF.1−/− mice that had been generated on the C57BL/6 background were challenged by infection with L. major, they showed significantly increased footpad swelling (Figure 2A) and a 20- to 100-fold higher parasite load in infected footpads and popliteal draining lymph nodes, compared with control mice (Figure 2B and C). Analyses of cytokine production of draining lymph node T cells after re-stimulation with L. major antigen revealed that virtually no IFNγ was produced from these cells deficient for BOB.1/OBF.1. In contrast, the TH2 cytokine IL4 was not produced by either wild-type or BOB.1/OBF.1−/− cells upon re-stimulation, whereas the IL13 production was low but detectable and found slightly increased in comparison to wild-type cells (Figure 2D). As BOB.1/OBF.1−/− lymphocytes were able to proliferate in response to L. major antigen, they had clearly been sensitized and were not anergic (Supplementary Figure S4). In addition, analyses of T-cell populations in draining lymph nodes of infected animals revealed again reduced numbers of B as well as T cells in BOB.1/OBF.1−/− mice (Supplementary Figure S5).
Figure 2.
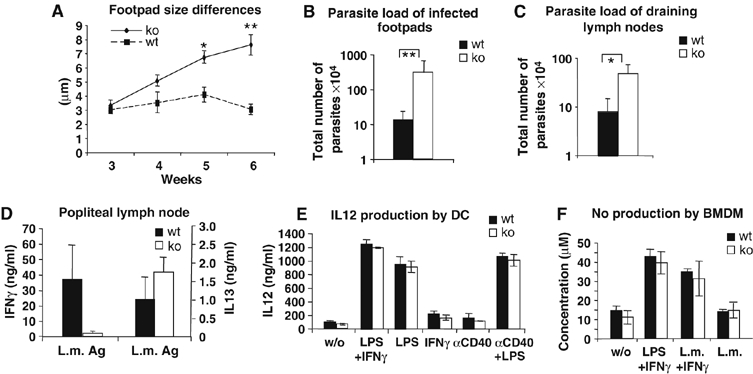
BOB.1/OBF.1−/− mice are not able to contain parasites after infection with L. major. (A) Left hind footpads of wild-type (n=6) and BOB.1/OBF.1−/− mice (n=6) were infected with L. major parasites. Differences between left and right hind footpad size were measured. *P⩽0.05; **P⩽0.005. After 6 weeks, mice were analyzed for (B) parasite load of infected footpads and for (C) parasite load of draining lymph nodes. (D) CD4+ T-cell numbers from draining lymph nodes were adjusted, re-plated and re-stimulated with L. major antigen. This experiment was performed with triplicates. After 24 h of stimulation, supernatants were analyzed for IFNγ (P=0.0056) and IL13 (P=0.078) levels by ELISA. Data were analyzed with Student's t-test. Infection experiments were performed twice with similar results. Data show mean values±s.d. (E) Bone marrow-derived BOB.1/OBF.1−/− DCs produce equal amounts of IL12 compared with wild-type DCs, after stimulation with the indicated inducers for 24 h. (F) BMDM were obtained from BOB.1/OBF.1−/− and wild-type mice killed after 6 weeks of L. major infection, and the NO production in response to indicated stimuli was determined. (L.m.=L. major antigen). The experiments in (E) and (F) were performed with triplicates and repeated twice. One representative experiment is shown. Data are the means±s.d.
As IL12 produced by DCs and macrophages is a major stimulus for generation of the TH1 response, we asked whether DCs and macrophages from BOB.1/OBF.1−/− mice show normal IL12 production. Bone marrow-derived DCs (Figure 2E) and macrophages (data not shown) from BOB.1/OBF.1−/− mice released normal amounts of IL12 in response to different stimuli. Furthermore, wild-type and BOB.1/OBF.1−/− bone marrow-derived macrophages from L. major infected animals showed equally effective production of NO after phagocytosis of L. major and activation with IFNγ and LPS in vitro (Figure 2F).
The inability of BOB.1/OBF.1−/− mice to cure L. major infection is intrinsic to CD4+ cells
To exclude that the impaired B-cell compartment of BOB.1/OBF.1−/− mice had an impact on susceptibility to L. major in our model, Rag2−/− mice were reconstituted with purified CD4+ cells from either wild-type or BOB.1/OBF.1−/− mice, together with wild-type B cells, and subsequently infected with L. major. Again, after 4 weeks footpad swelling was significantly larger in Rag2−/− mice reconstituted with CD4+ T cells from BOB.1/OBF.1−/− mice compared with Rag2−/− mice that had received wild-type CD4+ T cells (Figure 3A). This was accompanied by a 10- and 20-fold increase in parasite load of infected footpads and of draining lymph nodes in mice reconstituted with mutant CD4+ T cells (Figure 3B and C). Notably, non-reconstituted Rag2−/− mice showed a less than two-fold higher footpad swelling and parasite load in infected footpads than mice substituted with BOB.1/OBF.1−/− CD4+ T cells (Figure 3A and data not shown). In addition, the cell numbers of draining lymph nodes, as well as the ability of these cells to proliferate and to produce cytokines in response to L. major antigen ex vivo, was analyzed. After 2 weeks of infection, the general cell numbers as well as the numbers of CD4+ T cells in draining lymph nodes of infected Rag2−/− mice that received wild-type or BOB.1/OBF.1−/− mice were found to be equal. In addition, there were no differences in the ability of these cells to proliferate in response to L. major antigen, albeit these responses were very low. After 4 weeks of infection, we could detect a slight reduction of cell numbers in draining lymph nodes of mice that received BOB.1/OBF.1−/− CD4+ T cells (Supplementary Figures S6). In addition, the wild-type cells showed a two-fold higher proliferation potential and an approximately 100-fold increase in IFNγ secretion in comparison to BOB.1/OBF.1−/− cells, upon re-stimulation with L. major antigen (Figure 3D and Supplementary Figure S6). Thus, the defect to control L. major infection is indeed caused by impaired function of BOB.1/OBF.1−/− CD4+ T cells.
Figure 3.
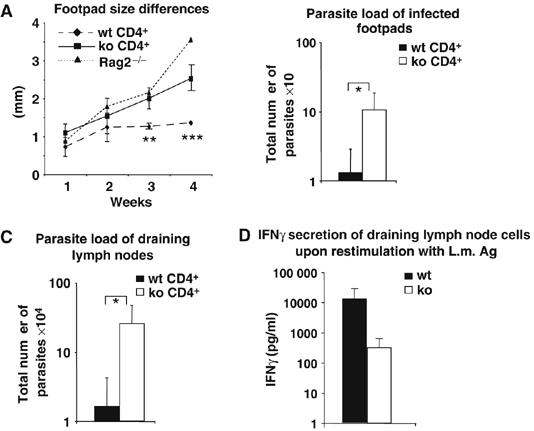
The inability of BOB.1/OBF.1−/− mice to develop an efficient response to L. major infection is T-cell intrinsic. Rag2−/− animals were reconstituted with either wild-type (n=10) or BOB.1/OBF.1−/− CD4+ T cells (n=8), together with wild-type B cells, and infected the next day with L. major. (A) Differences of left and right footpad sizes were measured. After 4 weeks, animals were killed and the parasite load of infected footpads (B) as well as of draining lymph nodes (C) was determined. *P⩽0.05; **P=0.039; ***P=0.009. Data were analyzed with Student's t-test. The results of three independent experiments are shown in (A). In two of three independent experiments the draining lymph nodes as well as the footpads were used for the analyses of parasite load (B, C); in the third experiment, the draining lymph node cells were used for the analysis of IFNγ secretion upon re-stimulation with L.m. antigen. (D) The numbers of draining lymph node CD4+ T cells were enumerated, equal amounts of CD4+ T cells were reseeded and re-stimulated with L. major antigen. After 6 days in culture, IFNγ production was measured by ELISA.
T helper cells generated from BOB.1/OBF.1−/− mice show defects in cytokine secretion
To clarify the molecular mechanisms responsible for the severe leishmaniasis observed in BOB.1/OBF.1−/− mice, we first asked whether the imbalanced TH1/TH2 cytokine production observed during infection with L. major could be reproduced in vivo. Therefore, we analyzed cytokine production in polarized and re-stimulated T helper cell subsets established from purified naive CD4+ cells. Naive CD4+ lymph node T cells were cultured either under neutral (TH0) conditions or polarized toward TH1 or TH2 lineages, respectively. After re-stimulation with αCD3/αCD28 antibodies, secreted cytokines were analyzed by ELISA. Like in our in vivo experiment, the expression of TH1 cytokines IL2 and IFNγ was reduced in BOB.1/OBF.1−/− cells (Figure 4A and 4B). In contrast, the synthesis of TH2 cytokines IL4 and IL13 was enhanced (Figure 4C and 4D). These differences in cytokine secretion by BOB.1/OBF.1−/− TH1 or Th2 cells were already detectable after 48–72 h of polarization initiation (Supplementary Figure S7).
Figure 4.
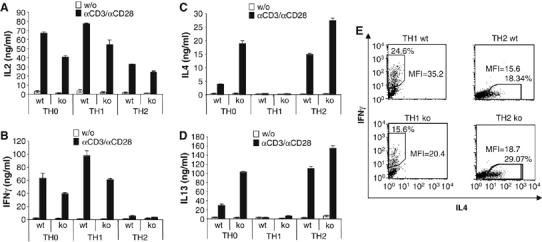
BOB.1/OBF.1 deficiency leads to impaired function of T helper cell subsets. Naïve CD4+ T from lymph nodes of wild-type and BOB.1/OBF.1−/− mice were grown either under neutral conditions or under polarization conditions. After 6 days, cells were washed, adjusted in cell numbers, re-plated and either left uninduced or re-stimulated with plate-bound αCD3+αCD28. After 24 h in culture, cytokine release was measured by ELISA. (A, B) BOB.1/OBF.1−/− TH0 and TH1 cells produce less IL2 and IFNγ in comparison with wild-type cells. (C, D) BOB.1/OBF.1−/− TH0 and TH2 cells produce increased levels of IL4 and IL13 in comparison with wild-type cells. The experiment was performed with triplicates and repeated at least three times. One representative experiment is shown. Data are the means±s.d. (E) IFNγ produced by TH1 cells or IL4 produced by TH2 cells established from naïve cells of wild-type (wt) or BOB.1/OBF.1−/− (ko) mice was analyzed by intracellular cytokine FACS analyses. MFI, mean fluorescence intensity.
To address the question whether the reduction in IFNγ levels produced by BOB.1/OBF.1−/− TH1 cells is caused by a lower IFNγ production per cell or by fewer cells producing IFNγ, we performed intracellular cytokine staining experiments. We observed that the number of cells staining positive for IFNγ was reduced. In addition, the amount of IFNγ produced per cell is reduced, as is evident from the lower mean fluorescence intensity. Analyses of TH2 cells established from BOB.1/OBF.1−/− mice revealed corresponding increases in cell numbers that produce IL4 and also in IL4 mean fluorescence intensity (Figure 4E).
The imbalanced secretion of TH1 and TH2 cytokines results from an altered mRNA expression
To analyze whether reduced IFNγ levels are a consequence of reduced RNA levels rather than defects in cytokine secretion, we quantified cytokine RNA levels by RT–PCR. This analysis showed reduced IFNγ RNA levels in polarized and re-stimulated BOB.1/OBF.1−/− TH0 and TH1 cells. In contrast, IL4 RNA levels were slightly increased in BOB.1/OBF.1−/− TH2 cells (Figure 5A). These data were confirmed by real-time PCR (Figure 5B). In addition, the expression of the key regulatory transcription factors for TH1 and TH2 development, T-bet and GATA-3, was analyzed and found to be unaffected in BOB.1/OBF.1−/− cells (Figure 5B and C). Analysis of the IL12 receptor β2 (IL12Rβ2) mRNA (Figure 5D), necessary for developing TH1 cells to receive the IL12 signal from APCs, also revealed a slight decrease in untreated and re-stimulated TH1 cells generated from BOB.1/OBF.1−/− mice. This reduced IL12Rβ2 expression levels might be physiologically relevant, as we saw a reduced polarization frequency toward TH1 cells in vitro. In addition, we observed lower IL12-induced IFNγ secretion by BOB.1/OBF.1-deficient TH1 cells in comparison with wild-type cells (data not shown).
Figure 5.
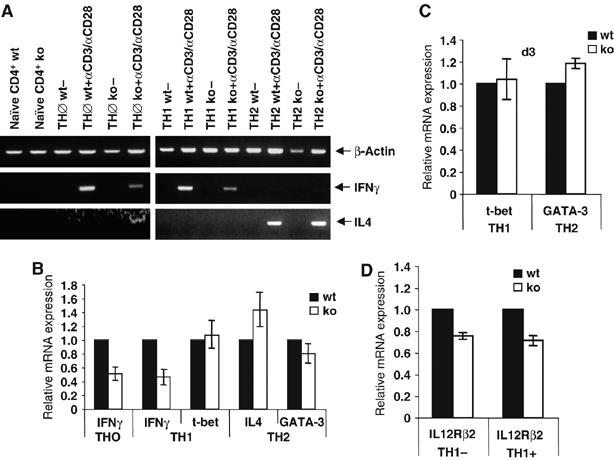
The imbalanced synthesis of TH1 and TH2 cytokines results from an altered mRNA expression. (A) Naïve CD4+ T cells were either left untreated or kept for 6 days either under neutral conditions (TH0) or in TH1 or TH2 differentiation medium, re-plated and either left untreated or induced with plate-bound αCD3+αCD28. After 24 h, RNA was prepared and analyzed by RT–PCR for IFNγ and IL4 mRNA expression. The analysis of β-actin expression served as internal control. (B) TH0, TH1 or TH2 cells were generated from naive CD4+ cells derived from wild-type and BOB.1/OBF.1−/− mice. After re-stimulation of cells for 24 h with plate-bound αCD3+αCD28, mRNA expression of IFNγ, IL4, T-bet, GATA-3 was analyzed by real-time PCR. (C) Expression of T-bet and GATA-3 was analyzed at day 3 (d3) of T helper cell differentiation. (D) Expression of IL-12Rβ2 mRNA was analyzed in differentiated TH1 cells from wild-type and BOB.1/OBF.1−/− cells that were either left untreated (−) or induced with plate-bound αCD3+αCD28 (+). Relative expression levels are shown. The experiments were performed with triplicates. Data are means±s.d.
BOB.1/OBF.1 directly activates the IFNγ promoter
Since BOB.1/OBF.1 influences as a transcriptional coactivator the activity of gene expression, we asked whether the reduced IFNγ expression observed in BOB.1/OBF.1−/− TH0 and TH1 cells might be due to a direct contribution of BOB.1/OBF.1 to the IFNγ promoter activity. BOB.1/OBF.1 regulates transcription together with Oct factors. Therefore, we searched for octamer motifs in the IFNγ promoter and found five potential sites within the upstream 900 bp (Supplementary Figure S8). All sites were analyzed by electrophoretic mobility shift assay (EMSA) for ternary complex formation with Oct1 and BOB.1/OBF.1. HeLa nuclear extracts supplemented with in vitro translated BOB.1/OBF.1 were used. The strongest ternary complex similar to that of a consensus octamer sequence was observed with the octamer site, close to the TATA box (M5; −129 to −136) (Figure 6A and Supplementary Figure S8). In supershift experiments, antibodies against Oct1 and BOB.1/OBF.1 blocked ternary complex formation. Oct2 antibodies did not interfere, since HeLa cells do not express Oct2 protein (Figure 6A). The IFNγ M5 site could also efficiently compete against a labelled consensus octamer site (Figure 6B). In contrast, when the M5 sequence was mutated, no binding of Oct and BOB.1/OBF.1 or competition could be detected (Figure 6B). A second more distal octamer motif (M2) (Figure 6A and Supplementary Figure S8) was also able to form ternary complexes, albeit with a reduced efficiency (Figure 6A). Both sites vary from the consensus Oct binding site at the first or last position, respectively. Oct proteins have also been described to bind to the murine IL2 and IL4 promoters. We therefore analyzed these sites for ternary complex formation. Indeed, weak complexes composed of Oct and BOB.1/OBF.1 proteins were detected with the IL2 promoter site, but no complex was detected at the IL4 promoter (Figure 6A).
Figure 6.
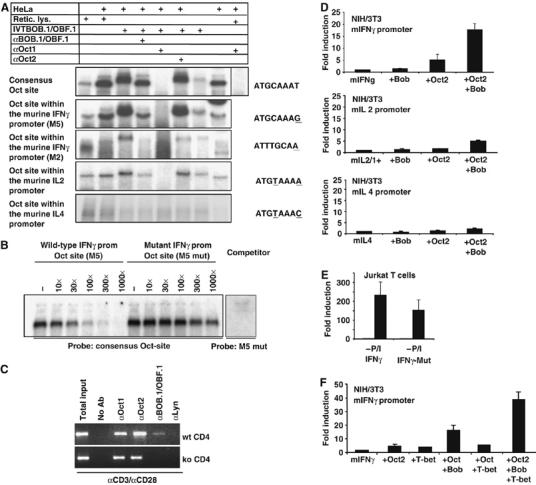
BOB.1/OBF.1 is involved in the regulation of the IFNγ promoter, in vitro as well in vivo. (A) EMSA experiments with HeLa nuclear extracts were performed using labelled oligonucleotides bearing either a consensus octamer motif or the octamer sequences identified within the IFNγ promoter (M5 and M2), or within the IL2 and IL4 promoter. Unprogrammed reticulocyte lysate or in vitro translated BOB.1/OBF.1 protein was included. Complexes were analyzed by adding specific antibodies for Oct1, Oct2 or BOB.1/OBF.1, as indicated. (B) Ternary complexes formed between the consensus octamer site and HeLa nuclear extracts supplemented with in vitro translated BOB.1/OBF.1 were challenged with either the wild-type or mutated sites from the IFNγ promoter (the octamer motif ATGCAAAT was mutated to AgtacAAT). The mutated site was also unable to form ternary complexes when tested as labeled fragment (right panel). (C) ChIP assay. The chromatin of αCD3+αCD28-induced wild-type or BOB.1/OBF.1−/− CD4+ T cells was analyzed. Immunoprecipitated DNA was PCR amplified using primers specific for the IFNγ promoter M5 octamer motif. Chromatin immunoprecipitated with αLyn antibodies was included as negative control. (D) Analysis of the dependence of the IFNγ promoter activity on Oct and BOB.1/OBF.1 proteins in transient transfection experiments using NIH/3T3 fibroblasts. The value of the IFNγ promoter activity without cotransfection was set to 1. The same transfection analyses were performed for the IL2 and IL4 promoters. The fold induction upon cotransfection of Oct2 and BOB.1/OBF.1 expression vectors was determined. (E) Jurkat T cells were transfected with either the wild-type or the octamer-mutated (M5 mut) IFNγ promoter reporter construct. Subsequently, cells were left untreated (−) or induced with PMA/ionomycin (+P/I). The activity of the unstimulated wild-type promoter was set to 1 and the fold induction was determined. (F) Oct2, BOB.1/OBF1 and T-bet transactivate the IFNγ promoter in synergy. Expression vectors for Oct2, BOB.1/OBF.1 and T-Bet were cotransfected along with the murine IFNγ promoter reporter construct into NIH/3T3 cells. The value of the IFNγ promoter activity without cotransfection was set to 1. The fold induction was determined. (D–F) The experiments were repeated five times and mean values as well as s.d. were determined.
To investigate whether BOB.1/OBF.1 binds to the IFNγ promoter in vivo, chromatin immunoprecipitation (ChIP) was performed with purified CD4+ T cells from wild-type or BOB.1/OBF.1−/− mice. Whereas binding of Oct1 and Oct2 was detectable in wild-type and BOB.1/OBF.1−/− cells, BOB.1/OBF.1 binding to the IFNγ promoter M5 octamer motif could be detected only in αCD3+αCD28 stimulated wild-type cells (Figure 6C). No binding of Oct proteins and BOB.1/OBF.1 to the IFNγ promoter octamer motif M2 could be detected in vivo (data not shown).
As we observed a binding of BOB.1/OBF.1 together with Oct to the IFNγ promoter, we wondered whether this binding results in the activation of IFNγ promoter activity. Therefore, the promoters of the IFNγ-, IL2- and IL4 genes were cloned in front of a luciferase reporter gene and transfected into NIH/3T3 cells, along with Oct2, BOB.1/OBF1 or a combinations of these expression vectors. Consistent with our binding studies, we observed that BOB.1/OBF.1, together with Oct2,was able to transactivate the murine IFNγ promoter by approximately 20-fold (Figure 6D). The IL2 promoter activity is also stimulated by BOB.1/OBF.1 together with Oct2, albeit to a lesser extent (five-fold). In contrast, IL4 promoter activity was virtually not affected by coexpression of BOB.1/OBF.1 and Oct2 (Figure 6D).
In order to elucidate the importance of the octamer motif for IFNγ promoter activity in T cells, Jurkat cells were transfected with either the wild-type or the mutant IFNγ luciferase reporter construct (M5 mut; Figure 6B). The mutated octamer motif causes a reduction of 35% of the PMA/ionomycin-induced IFNγ promoter activity in Jurkat T cells (Figure 6E). This indicates that this octamer motif and the interacting transcription factors contribute to full IFNγ promoter activity in T cells.
The T-box transcription factor T-bet is the major factor necessary for IFNγ production. Initially, T-bet binding sites were identified about 2300 bp upstream of the IFNγ promoter. Additionally, an alternative region, the T-bet responsive unit (−445 to −415), was described (Soutto et al, 2002). Recently, several T-bet binding sites were identified within the proximal IFNγ promoter region (Cho et al, 2003). Moreover, one of those sites overlaps with the M5 motif, identified in our study as an Oct/BOB.1/OBF.1 binding site (Supplementary Figure S8). Therefore, we asked, whether Oct and BOB.1/OBF.1 cooperate with T-bet to transactivate the IFNγ promoter. Transfection experiments revealed a synergistic effect of Oct and BOB.1/OBF.1, together with T-bet, on the IFNγ promoter activity when cotransfected into NIH/3T3 cells (Figure 6F).
In conclusion, these findings indicate that BOB.1/OBF.1 directly regulates IFNγ promoter activity in T cells together with Oct2 and T-bet.
BOB.1/OBF.1 influences PU.1 expression in TH2 cells
Given that we could not show a direct regulation of IL4 or IL13 promoter activity by BOB.1/OBF.1 (Figure 6A and D, data not shown), we wondered how the expression of these cytokines might be affected by the loss of BOB.1/OBF.1. Recent experiments demonstrated that the transcription factor PU.1 is expressed in TH2 cells and attenuates TH2 cytokine expression by interfering with GATA-3 function (Chang et al, 2005). Previous cotransfection experiments had suggested a direct regulation of PU.1 expression by Oct transcription factors and BOB.1/OBF.1 in B cells (Kistler et al, 1995; Chen et al, 1996). We therefore analyzed PU.1 expression in wild-type and BOB.1/OBF.1-deficient TH2 cells. PU.1 protein levels (Figure 7A) and RNA levels (Figure 7B) were clearly reduced in BOB.1/OBF.1-deficient cells. The PU.1 promoter contains a well-conserved octamer motif, and we could show that Oct1 and BOB.1/OBF.1 can form ternary complexes at this site (Figure 7C). When nuclear extracts from HeLa cells were incubated with in vitro translated BOB.1/OBF.1, a slower migrating ternary complex was detected in an EMSA (Figure 7C, lane 3). Supershift assays revealed that this complex was composed of BOB.1/OBF.1 and Oct1 (Figure 7C, lanes 4 and 5). This complex formation at the octamer motif of the PU.1 promoter was identical to that formed at the consensus octamer site (Figure 7C). In addition, transfection of Oct2 and BOB.1/OBF.1 expression vectors along with a reporter consisting the PU.1 promoter revealed a synergistic stimulation of the PU.1 promoter (Figure 7D). Mutating the octamer motif causes a reduction by 65% of the PMA/ionomycin-induced PU.1 promoter activity in T cells (Figure 7E). Finally, in order to prove the contribution of Octamer factors and BOB.1/OBF.1 in the regulation of the PU.1 promoter in vivo, ChIP assays were performed using stimulated TH1 or TH2 cells. In those experiments, a direct binding of Oct factors as well as of BOB.1/OBF.1 to the PU.1 promoter in wild-type TH2 cells could be demonstrated. In contrast, in TH1 cells no binding of the analyzed transcription factors was observed (Figure 7F). Together, these data demonstrate the importance of Oct and BOB.1 proteins for PU.1 promoter activity in T cells. Reduced PU.1 levels most likely result in higher GATA-3 activity and consequently increased TH2 cytokine expression levels.
Figure 7.
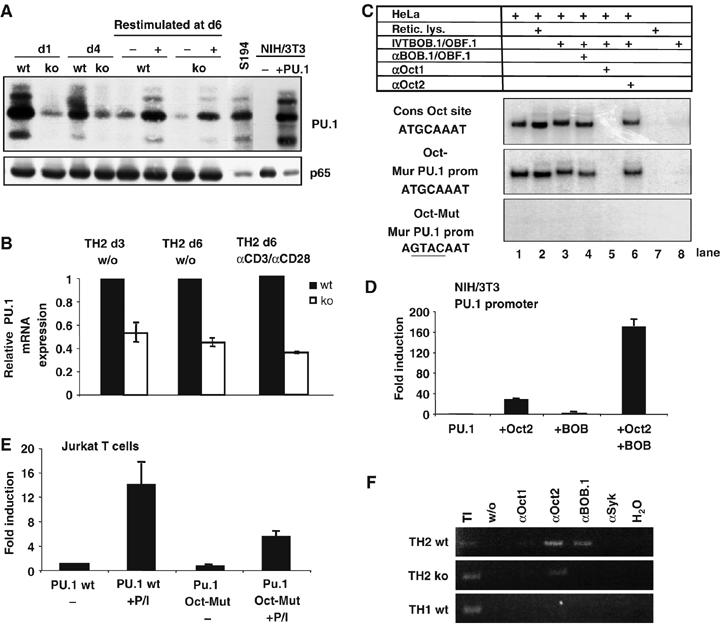
BOB.1/OBF.1 expression is necessary for PU.1 promoter activity in TH2 cells. (A) PU.1 expression is downregulated in developing and established BOB.1/OBF.1−/− TH2. Wild-type or BOB.1/OBF.1−/− developing TH2 cells were analyzed for PU.1 expression at day 1 (d1), day 4 (d4) and day 6 (d6) during differentiation. At day 6, cells were re-stimulated for 24 h with plate-bound αCD3+αCD28. The analyses of extracts from the S194 B-cell line or from NIH/3T3 cells, either left untreated (−) or transfected with a murine PU.1 expression vector (+PU.1), served as internal controls. Analyses of p65 expression served as loading control. (B) RNA was prepared from developing TH2 cells at day 3 (d3) or differentiated TH2 cells at day 6 (d6), or from re-stimulated, as indicated, at d6 established TH2 cells from either wild-type or BOB.1/OBF.1−/− mice and analyzed by real-time PCR for relative PU.1 expression. The experiments were performed in triplicates. Data are the means±s.d. (C) Oct and BOB.1/OBF.1 form ternary complexes at the octamer motif of the PU.1 promoter (see Figure 6A). (D) Oct and BOB.1/OBF.1 synergistically transactivate the PU.1 promoter (see Figure 6D). (E) The octamer motif is essential for the induction of the PU.1 promoter activity in T cells. Reporter constructs bearing either the wild-type or octamer mutant PU.1 promoter were transfected into Jurkat T cells and subsequently stimulated with PMA/ionomycin. The activity of the wild-type promoter without induction was set to 1. The fold induction was determined. (D, E) The experiments were repeated five times and mean values±s.d. are shown. (F) ChIP assay. The chromatin of αCD3+αCD28-induced wild-type or BOB.1/OBF.1−/− TH1 and TH2 cells was analyzed. Immunoprecipitated DNA was PCR amplified using primers specific for the PU.1 promoter octamer motif. Chromatin immunoprecipitated with αSyk antibodies was included as negative control.
Discussion
Earlier analyses of BOB.1/OBF.1−/− mice had revealed its importance for effective B-cell immune functions. Here, we show that the lack of the transcriptional coactivator BOB.1/OBF.1 in mice leads to an impaired TH1/TH2 balance. These effects are of decisive importance for the immune response in vivo. BOB.1/OBF.1−/− mice are not able to control experimental leishmaniasis, as reflected by increased parasite load of infected footpads and draining lymph nodes. Our new results now document that in addition to the defective B-cell effector functions, T-cell functions are also severely compromised in BOB.1/OBF.1−/− mice. This emphasizes the critical role that BOB.1/OBF.1 plays for the majority of effector cell populations of the adaptive immune system.
Lymphocytes express two octamer factors, the ubiquitously expressed Oct1 and the lymphocyte-specific Oct2 protein (Kamps et al, 1990; Schöler, 1991; Staudt and Lenardo, 1991). Whereas in B cells, both factors are constitutively expressed, in peripheral T cells as well as in non-transformed T-cell lines, Oct2 expression as well as BOB.1/OBF.1 expression and function are induced upon TCR stimulation (Kang et al, 1992; Zwilling et al, 1997). Here, we show that BOB.1/OBF.1 expression is detectable in polarized TH1 and TH2 cells, and that its expression levels can be further increased by stimulation of T helper cells.
Using an in vivo model of experimental leishmaniasis we found that BOB.1/OBF.1−/− mice, generated on the resistant C57BL/6 background, developed a severe infection with a drastically reduced capacity to contain the parasites. Importantly, whereas the IFNγ secretion in vitro was reduced only 30–40%, popliteal lymph node cells from L. major-infected animals were almost unable to produce IFNγ. These T cells from infected BOB.1/OBF.1−/− mice, however, were sensitized in an antigen-specific manner, since they proliferated in response to L. major antigen. In parallel, we found low but increased production of the TH2 cytokine IL13 in draining lymph node cells of infected animals, most likely a result of the reduced PU.1 expression found in BOB.1/OBF.1−/− TH2 cells. However, this increase was modest and we were unable to detect IL4 in draining lymph nodes of infected wild-type and BOB.1/OBF.1-deficient animals, or from reconstituted Rag2−/− mice after re-stimulation ex vivo with L. major antigen. This indicates that the immune response in BOB.1/OBF.1-deficient mice is not completely shifted toward a TH2 response. Therefore, we conclude that the reason for the severe leishmaniasis in BOB.1/OBF.1-deficient mice is primarily impaired IFNγ production.
No defects were found in DCs and macrophages that are involved in effective immune response against L. major. Despite the finding that B cells are not necessary for the elimination of L. major after infection with high parasite numbers (Sacks et al, 1984; Brown and Reiner, 1999), an effect of B cells in the activation of macrophages could not be excluded a priori. However, adoptive transfer experiments confirmed that the defect in BOB.1/OBF.1−/− mice is intrinsic to T cells.
We could show that BOB.1/OBF.1 deficiency had an effect on TH1 cell function. However, a direct regulation of the IFNγ promoter by Oct/BOB.1/OBF.1 had not been reported before. The promoter region encompassing roughly the first 600 bp upstream of the start site of transcription was identified to be responsible for signal- and tissue-specific control of IFNγ gene expression (Chrivia et al, 1990; Ciccarone et al, 1990; Penix et al, 1993). We identified several potential binding sites for Oct1/2 and BOB.1/OBF.1 within this region. A combination of in vitro and in vivo experiments documented the importance of these factors for IFNγ promoter activity. Binding of Oct2 and BOB.1/OBF.1 to a non-consensus octamer motif was also recently demonstrated for the Btk promoter (Brunner and Wirth, 2006). Moreover, we showed that Oct and BOB.1/OBF.1 act in synergy with T-bet in the IFNγ promoter activation. Together, our findings suggest that Oct transcription factors and BOB.1/OBF.1 are needed for high levels IFNγ gene expression.
We also saw reduced expression of the IL12Rβ2 in BOB.1/OBF.1−/− TH1 cells. APC-derived IL12 plays a key role in TH1 differentiation, as shown by the fact that mice deficient in either IL12 receptor or in IL12 itself show severe defects in TH1 responses (Magram et al, 1996; Wu et al, 1997, 2000). Additionally, although IL12 is the major cytokine necessary for TH1 cell generation, IFNγ secreted by CD4+ cells induces TH1 cell development via an autocrine loop, together with IL12 (Bradley et al, 1996). Consistent with the reduced expression of the IL12Rβ2 chain, we found reduced (40%) levels of IFNγ secretion upon IL12 stimulation (data not shown). As this reduction is similar to that seen after CD3/CD28 stimulation, the specific contribution of the reduced expression of IL12Rβ chain is unclear.
The importance of the octamer motif for IL2 transcription in T cells is well documented (Brunvand et al, 1988; Shibuya and Taniguchi, 1989; Hentsch et al, 1992; de Grazia et al, 1994; Pfeuffer et al, 1994). Octamer transcription factors also bind to the IL4 promoter (Chuvpilo et al, 1993; Li-Weber et al, 1998). However, they were shown to exhibit only a minor or possibly even an inhibitory effect on IL4 gene transcription (Pfeuffer et al, 1994; Cron et al, 2001). We confirmed binding of octamer transcription factors to the IL2 promoter octamer motif. Moreover, we found that Oct1/2 and BOB.1/OBF.1 form ternary complexes at the IL2 promoter. Consistent with the reduced expression of IL2 in BOB.1/OBF.1−/− T cells, we found that BOB.1/OBF.1 induced the IL2 promoter together with Oct2. Reduced expression levels of IL2 might be responsible for the reduced numbers of T cells that we observed in BOB.1/OBF.1−/− mice.
In contrast, no significant direct contribution of BOB.1/OBF.1 to IL4 gene expression was evident from our analyses. No functional octamer motif was detected within the IL13 promoter. Indeed, we found that the effect of BOB.1/OBF.1 on TH2 cytokines is rather indirect. Here, we show that PU.1 is a direct target of BOB.1/OBF.1 in TH2 cells. PU.1 in turn negatively regulates TH2 cytokine secretion by interfering with GATA-3 binding to promoter DNA. Hence, silencing of PU.1 expression in TH2 cells results in increased TH2 cytokine secretion (Chang et al, 2005). Therefore, it is likely that BOB.1/OBF.1 influences TH2 cytokine synthesis indirectly by regulation PU.1 expression. Recently, regulation of the PU.1-related transcription factor Spi-B by BOB.1/OBF.1 was reported (Bartholdy et al, 2006). Expression levels of Spi-B were very low in TH2 cells and no clear dependency on BOB.1/OBF.1 could be detected.
In summary, our data demonstrate that in addition to its key role in B lymphocyte effector functions, BOB.1/OBF.1 also plays a critical role in fine-tuning TH1 and TH2 cytokine expression. The relevance of this fine-tuning is evident upon challenge of BOB.1/OBF.1-deficient mice with L. major. Therefore, BOB.1/OBF.1 is a pleiotropic regulator of effector functions in the adaptive immune system.
Materials and methods
Mice
C57BL/6 wild-type, BOB.1/OBF.1−/− or Rag2−/− mice on the same genetic background (5–8 weeks of age) were obtained from our breeding facility. Infected mice were kept under SPF conditions.
Cell purification of CD4+ T cells
For naïve CD4+ T-cell purification, single cell suspensions of lymph nodes were incubated with antibodies against B220, CD8 (hybridoma supernatants), DX5 and MHCII (BD). After complement lysis, T cells were purified on a lympholyte M gradient. CD44+, Mac1+ (BD) and Gr1+ (hybridoma supernatant) cells were removed by negative selection using magnetic sheep anti-rat IgG beads (Dynal). For purification of CD4+ T cells, lymph node cell suspensions were depleted for B220+ and CD8+ cell by complement lysis. The achieved purity of cell populations was higher than 95%.
Cell purification of B220+ cells
B220+ cells were purified from spleen single cell suspensions by complement lysis of CD4+, CD8+ and DX5+ cells, followed by a lympholyte M gradient. Subsequently, CD44+, Mac1+, Ter119+ and Gr1+ cells were removed by negative selection using magnetic sheep anti-rat IgG beads (Dynal). Antibodies used for purification were purchased from BD.
Cell stimulation
Cells were stimulated as follows: αCD3: 4 μg/ml (soluble or coated; BD); αCD28: 0.5 μg/ml (BD); PMA: 50 ng/ml; ionomycin: 500 ng/ml (all from Sigma); DCs: 0.3 × 105 cells/well+αCD3; IFNγ: 2 μg/ml (Biotrend); IL4: 2% of supernatant of IL4-producing cells.
DCs or macrophages were stimulated as follows: LPS: 10 ng/ml (Invivogene); IFNγ:·50 U/ml; the ratio of L. major antigen per cell number was 3:1; αCD40: 10% of supernatant of αCD40-producing hybridoma cells. Cells were stimulated with IFNγ for 48 h before harvesting; all other inducers, including again IFNγ, were added 24 h before harvesting supernatants for cytokine or NO quantification.
TH1/TH2 polarization
Purified naïve CD4+ T cells (0.5–1 × 105 cells/well; purity ⩾95%) were stimulated with coated αCD3 plus soluble αCD28 (BD) in RPMI medium supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, 50 μM β-ME) in the presence of IL2 (2% of supernatant of IL2-producing cells; 5 ng/ml) (=TH0 conditions). For TH1 polarization, IFNγ (2 μg/ml; Biotrend), IL12 (2 μg/ml; Biotrend), and αIL4 monoclonal antibodies (5 μg/ml; BD), were added. For TH2 polarization, IL4 (2% supernatant of cells; 5 ng/ml) and αIFNγ (5% supernatant of hybridoma cells) were added. At day 6, cells were washed twice with PBS and re-stimulated, as indicated, for additional 24 h.
Proliferation assay
T-cell proliferation was assessed by 3H-labelled thymidine incorporation. A total of 0.5 × 106–1 × 106 purified CD4+ T cells per well were seeded in a 96-well plate and grown at 37°C and 5% CO2 in complete RPMI medium (± stimuli). After 36 h of stimulation, 0.5 μCi/well 3H-labelled thymidine (Amersham) was added for additional 10 h. Cells were harvested using the Inotech cell harvester system. Incorporated radioactivity was measured using the Wallace 1450 Microbeta Plus liquid scintillation counter and expressed as c.p.m. per well.
Intracellular cytokine staining
Cytokine production was determined by intracellular staining as previously described (Bird et al, 1998).
Bone marrow-derived DCs and macrophages (BMDM)
Tibias and femurs were flushed using a 25-gauge needle. Bone marrow cells were washed twice and cultured (3 × 106 cells/5 ml) in medium supplemented with granulocyte macrophage-colony stimulating factor (GM-CSF) (5% of supernatant of GM-CSF-producing cells). Non-adherent cells were harvested on day 8 and used for stimulation. BMDM were obtained from femurs, as described previously (Sunderkotter et al, 1993).
ELISA
Cytokine release was detected in supernatants by ELISA. All antibodies were purchased from BD, except for αIL13 (R&D). The recombinant mouse cytokine standards were from Cell Concepts (IL2 and IL13), Biotrend (IFNγ), PreproTechEC LTD (IL4) and PreproTech (TEBU) (IL12 p40). Extinction was analyzed at 405/490 nm on microplate ELISA reader, using the SoftMaxPro 4.3 LC software.
NO quantification
Macrophages cultured in 24-well plates were primed for 24 h with 50 U/ml murine IFNγ (Cell Concepts). Subsequently, cells were stimulated with LPS (Sigma) and IFNγ as positive control, and with L. major (L. major:macrophage ratio 3:1) opsonized with 1 % normal mouse serum (DAKO). Twenty-four hours later, supernatants were analyzed for nitrite concentration at 540 nm in a microtiter plate assay, using Griess reagent (Sigma), as described (Ding et al, 1988).
L. major infection
L. major strain MHOM/IL/81/SE/BNI was passaged in BALB/c mice in vivo and grown in vitro in Schneider's Drosophila medium (Cambrex) at 25°C, as previously described (Sunderkotter et al, 1993; Schonlau et al, 2003). Experimental leishmaniasis was initiated by subcutaneous injection of 2 × 107 L. major promastigotes (stationary phase) in 20 μl PBS into the left hind footpad. The thickness of the infected and the contralateral uninfected footpad were measured weekly using a metric caliper (Oditest).
Determination of parasite load
The number of viable parasites in infected footpads and draining lymph nodes was evaluated by a limiting-dilution assay, as previously described (Titus et al, 1985; Schonlau et al, 2003).
Adoptive transfer experiments
CD4+ T cells or B220+ B cells were purified from lymph nodes or spleen, respectively, as described above. Enrichment after depletion was 95–98% for the desired population. Age-matched Rag2−/− mice received 106 purified wild-type or BOB.1/OBF.1−/− CD4+ T and 107 wild-type B220+ cells intravenously. Twenty-four after the cell transfer into Rag2−/− mice, animals were infected with L. major.
EMSA
Whole-cell extract preparation and the EMSA procedures have been described (Lernbecher et al, 1993). The sequences of probes used are available upon request. For supershift experiments, an anti-Oct1 antibody (serum), anti-Oct2 antibody (C-20; Santa Cruz Biotechnology) or anti-BOB.1/OBF.1 antibody (serum) was used. EMSA conditions for detection of specific Oct/BOB.1/OBF.1 complexes were described previously (Luo et al, 1992). In these experiments, 2 μg HeLa extracts and 5 μl in vitro translated BOB.1/OBF.1 protein or the same amount of unprogrammed TNT lysates (Promega) were added where indicated.
Real-time PCR
Total RNA was isolated using the High pure RNA isolation Kit (Roche Diagnostics) and reverse transcribed using M-MLV reverse transcriptase. PCR primer sequences are available upon request. Real-time PCR was performed and analyzed as described (Boehm et al, 2001).
Transfection experiments
Transfections of NIH/3T3 cells were performed by electroporation (Bio-Rad) with 450 V and 250 μF in PBS; transfections of Jurkat cells were performed with 250 V and 975 μF in PBS.
Plasmids
The murine IFNγ promoter (+73 to –523) was cloned by genomic PCR. The fragment was cloned upstream of the luciferase reporter gene (Brunner et al, 2003a). Mutation of the octamer motif (M5; T2G, G3T, C4A, A5C) was generated using the QuickChange™ Mutagenesis kit (Promega) Expression vectors for BOB.1/OBF.1 have been described (Pfisterer et al, 1995). IL2, and IL4 promoter constructs were kindly provided by E Serfling. The cloning of the murine PU.1 promoter construct as well as the octamer mutant PU.1 promoter construct was described (Kistler et al, 1995). The expression vector for T-bet was kindly provided by M Boothby.
ChIP assay
The ChIP assay kit was used as described (Brunner et al, 2003a). Primers sequences for PCR were as follows:
IFNγ-M5 5′: ACAAGAATGGCACAGGTGGGC,
IFNγ-M5 3′: CCGAGGAGCTTCGATCAGGTA;
PU.1 5′: CAGCCGGCCAGAGACTTCCTG;
PU.1 3′: GCCTGCCACTGGGAGATAGTCC.
Supplementary Material
Supplementary Figure S1
Acknowledgments
We are grateful to K Missy, A Ushmorov and B Kistler for intensive discussions and scientific support, as well as P Weihrich, for excellent technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG, SFB 497) to TW (TP C5), to K-DF (TP C6), to AS (TP C7) and CB (TP C9), by the Interdisciplinary Research Center Ulm (project E9, CS), and by the Fonds der Chemischen Industrie to TW.
References
- Ansel KM, Lee DU, Rao A (2003) An epigenetic view of helper T cell differentiation. Nat Immunol 4: 616–623 [DOI] [PubMed] [Google Scholar]
- Bartholdy B, Du Roure C, Bordon A, Emslie D, Corcoran LM, Matthias P (2006) The Ets factor Spi-B is a direct critical target of the coactivator OBF-1. Proc Natl Acad Sci USA 103: 11665–11670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL (1998) Helper T cell differentiation is controlled by the cell cycle. Immunity 9: 229–237 [DOI] [PubMed] [Google Scholar]
- Boehm J, He Y, Greiner A, Staudt L, Wirth T (2001) Regulation of BOB.1/OBF.1 stability by SIAH. EMBO J 20: 4153–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley LM, Dalton DK, Croft M (1996) A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol 157: 1350–1358 [PubMed] [Google Scholar]
- Brown DR, Reiner SL (1999) Polarized helper-T-cell responses against Leishmania major in the absence of B cells. Infect Immun 67: 266–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner C, Laumen H, Nielsen PJ, Kraut N, Wirth T (2003a) Expression of the aldehyde dehydrogenase 2-like gene is controlled by BOB.1/OBF.1 in B lymphocytes. J Biol Chem 278: 45231–45239 [DOI] [PubMed] [Google Scholar]
- Brunner C, Marinkovic D, Klein J, Samardzic T, Nitschke L, Wirth T (2003b) B cell-specific transgenic expression of Bcl2 rescues early B lymphopoiesis but not B cell responses in BOB.1/OBF.1-deficient mice. J Exp Med 197: 1205–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner C, Wirth T (2006) Btk expression is controlled by Oct and BOB.1/OBF.1. Nucleic Acids Res 34: 1807–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunvand MW, Schmidt A, Siebenlist U (1988) Nuclear factors interacting with the mitogen-responsive regulatory region of the interleukin-2 gene. J Biol Chem 263: 18904–18910 [PubMed] [Google Scholar]
- Chang HC, Zhang S, Thieu VT, Slee RB, Bruns HA, Laribee RN, Klemsz MJ, Kaplan MH (2005) PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity 22: 693–703 [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang P, Radomska HS, Hetherington CJ, Zhang DE, Tenen DG (1996) Octamer binding factors and their coactivator can activate the murine PU.1 (spi-1) promoter. J Biol Chem 271: 15743–15752 [DOI] [PubMed] [Google Scholar]
- Cho JY, Grigura V, Murphy TL, Murphy K (2003) Identification of cooperative monomeric Brachyury sites conferring T-bet responsiveness to the proximal IFN-gamma promoter. Int Immunol 15: 1149–1160 [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Wedrychowicz T, Young HA, Hardy KJ (1990) A model of human cytokine regulation based on transfection of gamma interferon gene fragments directly into isolated peripheral blood T lymphocytes. J Exp Med 172: 661–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuvpilo S, Schomberg C, Gerwig R, Heinfling A, Reeves R, Grummt F, Serfling E (1993) Multiple closely-linked NFAT/octamer and HMG I(Y) binding sites are part of the interleukin-4 promoter. Nucleic Acids Res 21: 5694–5704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone VC, Chrivia J, Hardy KJ, Young HA (1990) Identification of enhancer-like elements in human IFN-gamma genomic DNA. J Immunol 144: 725–730 [PubMed] [Google Scholar]
- Cippitelli M, Sica A, Viggiano V, Ye J, Ghosh P, Birrer MJ, Young HA (1995) Negative transcriptional regulation of the interferon-gamma promoter by glucocorticoids and dominant negative mutants of c-Jun. J Biol Chem 270: 12548–12556 [DOI] [PubMed] [Google Scholar]
- Cron RQ, Zhou B, Brunvand MW, Lewis DB (2001) Octamer proteins inhibit IL-4 gene transcription in normal human CD4T cells. Genes Immun 2: 464–468 [DOI] [PubMed] [Google Scholar]
- de Grazia U, Felli MP, Vacca A, Farina AR, Maroder M, Cappabianca L, Meco D, Farina M, Screpanti I, Frati L, Gulino A (1994) Positive and negative regulation of the composite octamer motif of the interleukin 2 enhancer by AP-1, OCT-2, and retinoic acid receptor. J Exp Med 180: 1485–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding AH, Nathan CF, Stuehr DJ (1988) Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol 141: 2407–2412 [PubMed] [Google Scholar]
- Gstaiger M, Knoepfel L, Georgiev O, Schaffner W, Hovens CM (1995) A B-cell coactivator of octamer-binding transcription factors. Nature 373: 360–362 [DOI] [PubMed] [Google Scholar]
- Hentsch B, Mouzaki A, Pfeuffer I, Rungger D, Serfling E (1992) The weak, fine-tuned binding of ubiquitous transcription factors to the Il-2 enhancer contributes to its T cell-restricted activity. Nucleic Acids Res 20: 2657–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J, Nielsen PJ, Fischer KD, Bujard H, Wirth T (2001) The B lymphocyte-specific coactivator BOB.1/OBF.1 is required at multiple stages of B-cell development. Mol Cell Biol 21: 1531–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps MP, Corcoran L, LeBowitz JH, Baltimore D (1990) The promoter of the human interleukin-2 gene contains two octamer-binding sites and is partially activated by the expression of Oct-2. Mol Cell Biol 10: 5464–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S-M, Tsang W, Doll S, Scherle P, Ko H-S, Tran A-C, Lenardo MJ, Staudt LM (1992) Induction of the POU domain transcription factor Oct-2 during T-cell activation by cognate antigen. Mol Cell Biol 12: 3149–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Qin F-F, Gong S, Stevens S, Luo Y, Nussenzweig M, Roeder RG (1996) The B-cell-specific transcription coactivator OCA-B/OBF-1/Bob-1 is essential for normal production of immunoglobulin isotypes. Nature 383: 542–547 [DOI] [PubMed] [Google Scholar]
- Kistler B, Pfisterer P, Wirth T (1995) Lymphoid- and myeloid-specific activity is determined by the combinatorial action of octamer and ets transcription factors. Oncogene 11: 1095–1106 [PubMed] [Google Scholar]
- Lernbecher T, Müller U, Wirth T (1993) Distinct NF-kB/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature 365: 767–770 [DOI] [PubMed] [Google Scholar]
- Li-Weber M, Salgame P, Hu C, Davydov IV, Laur O, Klevenz S, Krammer PH (1998) Th2-specific protein/DNA interactions at the proximal nuclear factor-AT site contribute to the functional activity of the human IL-4 promoter. J Immunol 161: 1380–1389 [PubMed] [Google Scholar]
- Liew FY (2002) T(H)1 and T(H)2 cells: a historical perspective. Nat Rev Immunol 2: 55–60 [DOI] [PubMed] [Google Scholar]
- Luo Y, Fujii H, Gerster T, Roeder RG (1992) A novel B cell-derived coactivator potentiates the activation of immunoglobulin promoters by octamer-binding transcription factors. Cell 71: 231–241 [DOI] [PubMed] [Google Scholar]
- Magram J, Sfarra J, Connaughton S, Faherty D, Warrier R, Carvajal D, Wu CY, Stewart C, Sarmiento U, Gately MK (1996) IL-12-deficient mice are defective but not devoid of type 1 cytokine responses. Ann NY Acad Sci 795: 60–70 [DOI] [PubMed] [Google Scholar]
- Marafioti T, Ascani S, Pulford K, Sabattini E, Piccioli M, Jones M, Zinzani PL, Delsol G, Mason DY, Pileri SA (2003) Expression of B-lymphocyte-associated transcription factors in human T-cell neoplasms. Am J Pathol 162: 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi H (2001) Octamer transcription factors upregulate the expression of CCR5, a coreceptor for HIV-1 entry. J Biol Chem 276: 8639–8642 [DOI] [PubMed] [Google Scholar]
- Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL (2000) Signaling and transcription in T helper development. Annu Rev Immunol 18: 451–494 [DOI] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL (2002) The lineage decisions of helper T cells. Nat Rev Immunol 2: 933–944 [DOI] [PubMed] [Google Scholar]
- Nielsen PJ, Georgiev O, Lorenz B, Schaffner W (1996) B lymphocytes are impaired in mice lacking the transcriptional co-activator Bob1/OCA-B/OBF1. Eur J Immunol 26: 3214–3218 [DOI] [PubMed] [Google Scholar]
- Penix L, Weaver WM, Pang Y, Young HA, Wilson CB (1993) Two essential regulatory elements in the human interferon gamma promoter confer activation specific expression in T cells. J Exp Med 178: 1483–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer I, Klein-Heßling S, Heinfling A, Chuvpilo S, Escher C, Brabletz T, Hentsch B, Schwarzenbach H, Matthias P, Serfling E (1994) Octamer factors exert a dual effect on the IL-2 and IL-4 promoters. J.Immunol 153: 5572–5585 [PubMed] [Google Scholar]
- Pfisterer P, Zwilling S, Hess J, Wirth T (1995) Functional characterization of the murine homolog of the B-cell-specific coactivator BOB.1/OBF.1. J Biol Chem 270: 29870–29880 [DOI] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N (2002) The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2: 845–858 [DOI] [PubMed] [Google Scholar]
- Sacks DL, Scott PA, Asofsky R, Sher FA (1984) Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol 132: 2072–2077 [PubMed] [Google Scholar]
- Samardzic T, Marinkovic D, Nielsen PJ, Nitschke L, Wirth T (2002) BOB.1/OBF.1 deficiency affects marginal-zone B-cell compartment. Mol Cell Biol 22: 8320–8331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter P, Matthias P (1997) The B cell-specific coactivator OBF-1 (OCA-B, Bob-1) is inducible in T cells and its expression is dispensable for IL-2 gene induction. Immunobiology 198: 207–216 [DOI] [PubMed] [Google Scholar]
- Schöler HR (1991) Octamania: the POU factors in murine development. Trends Genet 7: 323–329 [DOI] [PubMed] [Google Scholar]
- Schonlau F, Schlesiger C, Ehrchen J, Grabbe S, Sorg C, Sunderkotter C (2003) Monocyte and macrophage functions in M-CSF-deficient op/op mice during experimental leishmaniasis. J Leukoc Biol 73: 564–573 [DOI] [PubMed] [Google Scholar]
- Schubart DB, Rolink A, Kosco-Vilbois MH, Botteri F, Matthias P (1996) B-cell-specific coactivator OBF-1/OCA-B/Bob1 required for immune response and germinal centre formation. Nature 383: 538–542 [DOI] [PubMed] [Google Scholar]
- Schubart DB, Rolink A, Schubart K, Matthias P (2000) Cutting edge: lack of peripheral B cells and severe agammaglobulinemia in mice simultaneously lacking Bruton's tyrosine kinase and the B cell- specific transcriptional coactivator OBF-1. J Immunol 164: 18–22 [DOI] [PubMed] [Google Scholar]
- Shibuya H, Taniguchi T (1989) Identification of multiple cis-elements and trans-acting factors involved in the induced expression of human IL-2 gene. Nucleic Acids Res 17: 9173–9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, Young HA (1997) Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem 272: 30412–30420 [DOI] [PubMed] [Google Scholar]
- Siegel R, Kim U, Patke A, Yu X, Ren X, Tarakhovsky A, Roeder RG (2006) Nontranscriptional regulation of SYK by the coactivator OCA-B is required at multiple stages of B cell development. Cell 125: 761–774 [DOI] [PubMed] [Google Scholar]
- Soutto M, Zhang F, Enerson B, Tong Y, Boothby M, Aune TM (2002) A minimal IFN-gamma promoter confers Th1 selective expression. J Immunol 169: 4205–4212 [DOI] [PubMed] [Google Scholar]
- Staudt LM, Lenardo MJ (1991) Immunoglobulin gene transcription. Annu Rev Immunol 9: 373–398 [DOI] [PubMed] [Google Scholar]
- Strubin M, Newell JW, Matthias P (1995) OBF-1, a novel B cell-specific coactivator that stimulates immunoglobulin promoter activity through association with octamer proteins. Cell 80: 497–506 [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Kunz M, Steinbrink K, Meinardus-Hager G, Goebeler M, Bildau H, Sorg C (1993) Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J Immunol 151: 4891–4901 [PubMed] [Google Scholar]
- Sweetser MT, Hoey T, Sun YL, Weaver WM, Price GA, Wilson CB (1998) The roles of nuclear factor of activated T cells and ying-yang 1 in activation-induced expression of the interferon-gamma promoter in T cells. J Biol Chem 273: 34775–34783 [DOI] [PubMed] [Google Scholar]
- Titus RG, Marchand M, Boon T, Louis JA (1985) A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol 7: 545–555 [DOI] [PubMed] [Google Scholar]
- Tong Y, Aune T, Boothby M (2005) T-bet antagonizes mSin3a recruitment and transactivates a fully methylated IFN-{gamma} promoter via a conserved T-box half-site. Proc Natl Acad Sci USA 102: 2034–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Ferrante J, Gately MK, Magram J (1997) Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J Immunol 159: 1658–1665 [PubMed] [Google Scholar]
- Wu C, Wang X, Gadina M, O'Shea JJ, Presky DH, Magram J (2000) IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J Immunol 165: 6221–6228 [DOI] [PubMed] [Google Scholar]
- Yang J, Murphy TL, Ouyang W, Murphy KM (1999) Induction of interferon-gamma production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur J Immunol 29: 548–555 [DOI] [PubMed] [Google Scholar]
- Ye J, Cippitelli M, Dorman L, Ortaldo JR, Young HA (1996) The nuclear factor YY1 suppresses the human gamma interferon promoter through two mechanisms: inhibition of AP1 binding and activation of a silencer element. Mol Cell Biol 16: 4744–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang L, Luo Y, Roeder RG (2001) Identification and characterization of a novel OCA-B isoform. Implications for a role in B cell signaling pathways. Immunity 14: 157–167 [PubMed] [Google Scholar]
- Zhang F, Wang DZ, Boothby M, Penix L, Flavell RA, Aune TM (1998) Regulation of the activity of IFN-gamma promoter elements during Th cell differentiation. J Immunol 161: 6105–6112 [PubMed] [Google Scholar]
- Zwilling S, Dieckmann A, Pfisterer P, Angel P, Wirth T (1997) Inducible expression and phosphorylation of coactivator BOB.1/OBF.1 in T cells. Science 277: 221–225 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1


