Abstract
COMATOSE (CTS) encodes a peroxisomal ATP-binding cassette transporter required not only for β-oxidation of storage lipids during germination and establishment, but also for biosynthesis of jasmonic acid and conversion of indole butyric acid to indole acetic acid. cts mutants exhibited reduced fertilization, which was rescued by genetic complementation, but not by exogenous application of jasmonic acid or indole acetic acid. Reduced fertilization was also observed in thiolase (kat2-1) and peroxisomal acyl-Coenzyme A synthetase mutants (lacs6-1,lacs7-1), indicating a general role for β-oxidation in fertility. Genetic analysis revealed reduced male transmission of cts alleles and both cts pollen germination and tube growth in vitro were impaired in the absence of an exogenous carbon source. Aniline blue staining of pollinated pistils demonstrated that pollen tube growth was affected only when both parents bore the cts mutation, indicating that expression of CTS in either male or female tissues was sufficient to support pollen tube growth in vivo. Accordingly, abundant peroxisomes were detected in a range of maternal tissues. Although γ-aminobutyric acid levels were reduced in flowers of cts mutants, they were unchanged in kat2-1, suggesting that alterations in γ-aminobutyric acid catabolism do not contribute to the reduced fertility phenotype through altered pollen tube targeting. Taken together, our data support an important role for β-oxidation in fertility in Arabidopsis (Arabidopsis thaliana) and suggest that this pathway could play a role in the mobilization of lipids in both pollen and female tissues.
In oilseed plants, fatty acids are stored in the seed as triacylglycerol (TAG), which is metabolized by lipase activity and peroxisomal β-oxidation to yield acetyl-CoA. Subsequent conversion of acetyl-CoA to succinate via the glyoxylate cycle provides energy and carbon skeletons, which are essential for seedling development before the capacity for photosynthesis is established (Baker et al., 2006). In each turn of the β-oxidation spiral, fatty acid chains are shortened by two carbon units with the concomitant generation of acetyl-CoA. Core reactions of the pathway are catalyzed by three enzymes: acyl-CoA oxidase (ACX), multifunctional protein (MFP), and 3-ketoacyl-CoA thiolase (KAT), each of which is encoded by more than one gene in Arabidopsis (Arabidopsis thaliana; for review, see Graham and Eastmond, 2002; Baker et al., 2006). Prior to β-oxidation, substrates must be activated by esterification to CoA and imported into the peroxisome. Because Arabidopsis contains a large family of acyl-activating enzymes, only a subset of which are located in the peroxisome, it is likely that both free acids and CoA esters could be transported across the peroxisomal membrane (Shockey et al., 2002, 2003; Fulda et al., 2004; Theodoulou et al., 2006).
COMATOSE (CTS; also known as PEROXISOME DEFECTIVE3 [PED3] and A. thaliana PEROXISOMAL ABC TRANSPORTER1 [AtPXA1]) encodes a peroxisomal ATP-binding cassette transporter, which was identified in a genetic screen for positive regulators of germination (Russell et al., 2000). cts-1 mutant seeds cannot germinate in the absence of classical dormancy-breaking treatments and do not establish in the absence of an exogenous energy source because of their inability to mobilize storage lipids by β-oxidation (Footitt et al., 2002, 2006). Mutant cts alleles have also been identified in screens for seedlings resistant to 2,4-dichlorophenoxybutyric acid and indole butyric acid (IBA; Zolman et al., 2001; Hayashi et al., 2002). These compounds are converted by one round of β-oxidation to the bioactive auxins, 2,4-dichlorophenoxyacetic acid and indole acetic acid (IAA), respectively, which stunt roots. These findings suggest that CTS either imports or regulates the peroxisomal import of a relatively wide range of substrates for β-oxidation.
We have shown recently that CTS also contributes to the synthesis of jasmonic acid (JA; Theodoulou et al., 2005). JA synthesis begins in the chloroplast, where membrane-derived linolenic acid (18:3) is converted to 12-oxophytodienoic acid (OPDA). OPDA exits the chloroplast and is imported into the peroxisome by a process requiring CTS, where it is reduced and then converted to JA by three cycles of β-oxidation (Schaller et al., 2004). JA levels are reduced, but not abolished, in cts mutants, indicating the existence of an additional, probably passive, route for uptake of JA precursors into the peroxisome (Theodoulou et al., 2005). Other authors have also demonstrated reduced JA in antisense lines or mutants in which β-oxidation is impaired; for example, plants deficient in KAT2, ACX1, or ACX5 (Cruz Castillo et al., 2004; Afitlhile et al., 2005; Li et al., 2005; Pinfield-Wells et al., 2005; Schilmiller et al., 2007). JA is important for male reproductive function in Arabidopsis, with roles in production of viable pollen, filament extension, and correct timing of anther dehiscence (Sanders et al., 2000; Stintzi and Browse, 2000). Unlike many JA biosynthetic and signaling mutants, however, cts mutants are male fertile. It is probable that the low residual levels of JA in cts mutants are sufficient for fertility because McConn and Browse (1996) used a triple fatty acid desaturase mutant with a leaky fad7 allele to demonstrate the existence of a threshold limit for the JA precursor, linolenic acid (18:3), and by extension, JA itself, for male fertility. Intriguingly, it has been reported that the double-mutant ped1,ped3-1, which lacks both CTS and KAT2, is sterile (Hayashi et al., 2002), perhaps because the compound effect of mutating both genes reduces JA levels below the fertility threshold. The apparently ubiquitous expression of CTS and the known role of JA in male fertility led us to examine in more detail the roles of this transporter during postgerminative growth to determine what impact loss of CTS function has on vegetative and reproductive processes.
Fertilization in flowering plants is a multistep process that requires delivery of pollen sperm cells to the ovules, which are located deep within the flower (Johnson and Preuss, 2002). Lipid metabolism and signaling potentially play important roles in several stages of fertilization, starting with production and release of viable pollen (Sanders et al., 2000; Stintzi and Browse, 2000) and synthesis of the protein- and lipid-rich pollen coat, which has a protective function and provides essential signals for binding, recognition, and hydration by the stigma (Zinkl et al., 1999). Deficiencies in pollen coat lipids lead to hydration defects and conditional male sterility in Arabidopsis cer mutants (Preuss et al., 1993; Fiebig et al., 2000) and pollen coat lipids have also been suggested to direct a water gradient, which is required for organization of cell polarity prior to germination (Wolters-Arts et al., 1998).
There are many parallels between pollen and seeds: both are dispersal units, which germinate to produce polarized, tip-growing structures, the pollen tube and the radicle, respectively. Both structures contain abundant presynthesized mRNAs, which are translated upon germination (Dure and Waters, 1965; Mascarenhas, 1993), although the biochemical mechanisms and regulation of pollen and seed germination appear to be different. In common with oilseeds, mature pollen of many species accumulates lipids (Baker and Baker, 1979). As in seeds, storage lipids take the form of TAGs, which are stored together with phospholipids and oleosins in oil bodies (Kuang and Musgrave, 1996; Kim et al., 2002). During pollen formation, oleosins and TAGs are synthesized in the abundant endoplasmic reticulum of tapetosomes and numerous oil droplets are produced in a manner apparently identical to that of seeds (Hsieh and Huang, 2005). Ultrastructural studies have reported the presence of numerous peroxisomes and lipid bodies in Arabidopsis pollen (Van Aelst et al., 1993). Synthesis of lipid bodies occurs shortly after pollen mitosis I and is restricted to the vegetative cell (Park and Twell, 2001). Microbodies, mitochondria, and lipid droplets are present in close spatial association in late pollen development and these organelles are also present in mature pollen, but they are more dispersed (Kuang and Musgrave, 1996). Similarly, pollen of the closely related species, oilseed rape (Brassica napus), has been shown to contain polymorphic microbodies, which were often in contact with lipid bodies, consistent with a function in lipid catabolism (Charzynska et al., 1989). In olive (Olea europea), oil bodies disappear following pollen germination (Rodriguez-Garcia et al., 2003) and, accordingly, a putative TAG lipase (SUGAR-DEPENDENT1 [SDP1]-like), which is abundantly expressed in pollen and flowers, has recently been identified in Arabidopsis (Eastmond, 2006). By analogy with SDP1, this gene might encode a TAG lipase involved in the mobilization of pollen oil reserves.
Following germination, the pollen tube must penetrate the cell wall of the stigma and grow into the style and transmitting tract. Pollen tubes grow at extremely high rates in vivo and consequently have a very high demand for energy (Lord, 2000; Lord and Russell, 2002). Because pollen is often in excess, tubes must compete for access to ovules; thus, rapid growth is key to male reproductive success (Howden et al., 1998). The pollen tube is known to interact intimately with the nutrient-rich extracellular matrix of the stylar tract and adhesion factors implicated in pollen tube growth have been identified in lily (Lilium longiflorum) and tobacco (Nicotiana tabacum; Lord, 2000, 2003; Lord and Russell, 2002). Pollen tube growth and guidance are separable genetically (Johnson et al., 2004), but the identities of diffusible signals directing pollen tubes to the ovules and ultimately permitting entry to the micropyle have remained elusive until recently (Johnson and Preuss, 2002; Higashiyama et al., 2003; McCormick and Yang, 2005). However, recent studies have implied that γ-aminobutyric acid (GABA) and NO are directional signals participating in pollen tube guidance in Arabidopsis (Palanivelu et al., 2003; Prado et al., 2004).
In this study, we have examined the postgerminative phenotype of cts mutants, with particular attention to fertility. We present data demonstrating that fertilization is compromised in cts and other β-oxidation mutants and that this defect is due to impaired pollen germination and reduced elongation of pollen tubes, rather than reduced JA or IAA biosynthesis. Although a role in processing of unknown signals cannot be ruled out, we propose that an important function of β-oxidation in fertilization is the provision of energy for pollen tube germination and growth via mobilization of TAG in both male and possibly also female tissues.
RESULTS
Postgerminative Phenotype of cts Mutants
Following mechanical rupture of the testa and seedling establishment in the presence of exogenous Suc, cts mutants can be transferred to soil and complete the life cycle (Russell et al., 2000; Footitt et al., 2002). However, mutation of CTS had subtle effects on vegetative growth: Rosette leaf number and area were reduced in cts-1 mutants compared to the wild type, Landsberg erecta (Ler), but these parameters were much less affected in cts-2 mutants, which more closely resembled the wild type, Wassilewskija2 (Ws2; Supplemental Fig. S1). Cauline leaf number and area were also reduced in cts-1 (Supplemental Fig. S1). Despite the reduction in leaf tissue in cts-1, there was no significant effect on photosynthesis in either of the mutant alleles (data not shown).
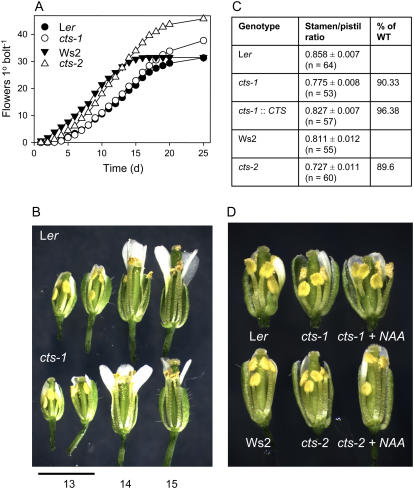
Although cts mutants were fertile, both cts-1 and cts-2 exhibited an altered reproductive phenotype. Time to bolting was not greatly affected, but both cts-1 and cts-2 alleles produced more flowers on the primary inflorescence than their respective wild types, Ler and Ws2 (Fig. 1A). Floral development was examined in the cts mutants: Mutant flowers appeared morphologically normal, with the exception that extension of the filaments was transiently delayed. Observation of flowers at stage 13 revealed that the ratio of long stamen to pistil length of the cts mutants was approximately 90% of the wild-type ratio (Fig. 1, B and C). This ratio was restored in cts-1 plants expressing the CTS open reading frame (ORF) under the control of the native CTS promoter (Fig. 1C). Filament extension in mutant flowers increased in subsequent developmental stages such that no difference between wild type and mutants was distinguishable. By floral stage 14, mutant anthers had extended above the stigma, permitting the deposition of pollen. Pollen of cts mutants was 100% viable, as judged by vital staining (data not shown).
Figure 1.
Floral phenotype of cts mutants. A, Cumulative flowering on the primary inflorescence (n = 23–32). B, Photograph of partially dissected flowers (stages 13–15) from primary inflorescences of Arabidopsis, accession Ler, and the cts-1 mutant. C, Ratio of long stamen/pistil lengths in different genotypes, including cts-1 plants transformed with a CTS promoter-cDNA cassette (cts-1∷CTS). Values are means ± se. D, Photograph of partially dissected flowers (stage 13) of wild-type (Ler; Ws2), cts mutants, and cts plants treated with 10 μm NAA.
A possible biochemical basis for delayed filament extension was investigated by application of hormones to cts flower buds. Whereas painting buds with JA did not affect filament extension (data not shown), this parameter was enhanced by spraying with the synthetic auxin analog, 1-naphthaleneacetic acid (NAA), such that treated mutants resembled the wild type (Fig. 1D). Exogenous IAA also increased extension of cts filaments, but a higher concentration was required (50 μm; data not shown), perhaps because IAA is less permeant and less stable in planta than NAA (Delbarre et al., 1996).
Fertilization Is Reduced in cts Mutants
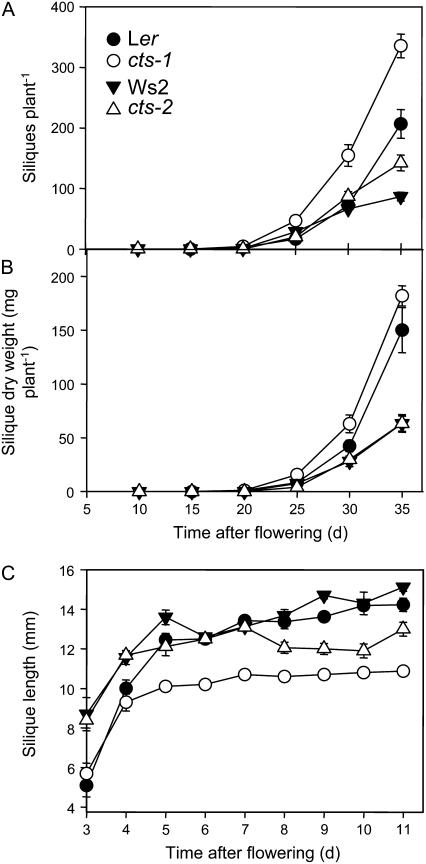
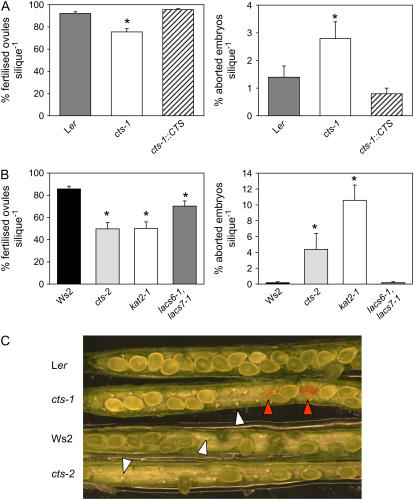
Following pollination, there was an approximate 50% increase in silique production in plants of both mutant alleles in comparison to their respective wild types (Fig. 2A). Total silique dry weight was unchanged in mutants, but mutant siliques were shorter than wild type (Fig. 2, B and C). Shorter siliques were associated with reduced fertilization of ovules and also increased abortion of embryos in mutant alleles (Fig. 3, A–C). The percentage of fertilized ovules was reduced from 92% in Ler to 76% in cts-1 and from 90% in Ws2 to 57% in cts-2. Wild-type levels of fertilization were restored by genetic complementation (Fig. 3A). To test whether the fertility defects were specific to cts mutants or reflected a more general defect in β-oxidation, fertilization was assessed in the single kat2-1 and the lacs6-1,lacs7-1 double mutants, which are deficient in KAT, and two peroxisomal long-chain acyl-CoA synthetases, respectively (Germain et al., 2001; Fulda et al., 2004). The percentages of fertilized ovules and aborted seeds in kat2-1 were similar to those in cts alleles, whereas the reduction in fertilization in lacs6-1,lacs7-1 was intermediate between that of cts alleles and wild type (Fig. 3B).
Figure 2.
Silique production in cts mutants and respective wild types. A, Total silique production. B, Total silique dry weight. C, Silique length. Black circles, Ler; white circles, cts-1. Black triangles, Ws2; white triangles, cts-2. Values are means ± se (n = 10–17).
Figure 3.
Fertilization in cts mutants. A, Percentage fertilized ovules and aborted embryos in siliques of Ler, cts-1, and cts-1 plants transformed with a CTS promoter-cDNA cassette (cts-1∷CTS). Siliques were harvested 10 DAF. Values are means ± se (n = 10–15 siliques). B, Percentage fertilized ovules and aborted embryos in siliques of wild type, Ws2, and β-oxidation mutant alleles: cts-2, kat2-1, lacs6-1,lacs7-1. The Ws4 wild-type behaved identically to Ws2 (data not shown). Values are means ± se (n = 11–18 siliques). Asterisks in A and B denote significant differences to wild type at 5%, as determined following ANOVA, using the lsds on the transformed data. C, Dissected siliques (harvested 10 DAF) showing unfertilized ovules (white arrowheads) and aborted embryos (red arrowheads) in cts mutants.
To investigate whether reduced fertility was a consequence of the reduced jasmonate levels found in cts mutants (Theodoulou et al., 2005), JA and its precursors, α-linolenic acid and OPDA, were painted onto the apical buds of the primary inflorescence of cts mutants and wild types. Application of α-linolenic acid did not affect wild types, but proved to be toxic to cts plants, causing scorching of flower buds, which is suggestive of impaired fatty acid metabolism in mutant flowers. JA and OPDA induced stunting of the inflorescence and shortening of siliques of cts plants, with no recovery of fertility. Wild-type plants were unaffected and application of the wetting agent, Tween 20, had no effect in any genotype (data not shown). Similarly, we tested whether fertility could be restored by application of exogenous auxin, but found no effect on fertilization following spraying of flower buds with 10 μm NAA.
cts Pollen Performs Less Well Than Wild-Type Pollen
Reduced fertility could be due to defects in either sporophytic tissue or in gametophytic tissue. To test for gametophytic effects of the cts mutation, plants heterozygous for cts-1 or cts-2 were allowed to self-fertilize and the genotypes of progeny were deduced by seed germination assays and allele-specific PCR. A significant deviation from the Mendelian ratio of 1:2:1 (wild type:+/cts:cts/cts) was observed (Table I), suggesting reduced transmission of mutant alleles (Howden et al., 1998). Therefore, the performance of gametes bearing the cts mutation was tested further in a series of reciprocal crosses between plants heterozygous for the cts-1 or cts-2 mutation and those homozygous for wild-type or mutant alleles. All crosses produced seeds that were subjected to germination assays and the genotype determined by PCR. The expected 1:1 segregation ratio of genotypes was observed in progeny of crosses between a heterozygous cts/+ female parent with either the wild type or with the homozygous mutant as the male parent (Table II). However, a highly significant deviation from this ratio was observed where heterozygous cts/+ pollen was used to pollinate wild-type or cts/cts pistils: approximately 5:1 for cts-1/+ pollen and 10:1 in the case of the cts-2/+ pollen (P < 0.001). Thus, transmission through the male, but not the female, gametes was affected (Table II). This result strongly suggests that, in a competitive situation, cts-1 and cts-2 pollen tubes are less able to target ovules than the respective wild types. The transmission efficiencies of cts alleles were also used to calculate the expected proportion of mutant progeny of selfed heterozygotes (Park et al., 1998), which agreed well with the observed values (Supplemental Table S1).
Table I.
Genetic transmission of cts alleles
Observed frequency of different genotypes in self-progeny of +/cts heterozygotes is shown.
| Self-Progeny of +/cts | ||
|---|---|---|
| +/+ | +/cts-1 | cts-1/cts-1 |
| 123 | 67 | 21 |
| +/+ | +/cts-2 | cts-2/cts-2 |
| 95 | 106 | 17 |
Table II.
Offspring ratios and transmission efficiencies determined from reciprocal crosses of plants carrying cts-1, cts-2, and CTS alleles
P, Probability of obtaining the observed variation from an expected 1:1 ratio by chance. P was calculated using GenStat (χ2 test). Transmission efficiency (TE) represents the fraction of mutant gametes that successfully transmit the mutation (Howden et al., 1998) and is defined as TE (%) = (no. mutants)/(no. wild-type plants) × 100.
| ♀ Parent | ♂ Parent | No. Offspring
|
Probability | TE | ||
|---|---|---|---|---|---|---|
| +/+ | cts-1/+ | cts-1/cts-1 | ||||
| +/+ | cts-1/+ | 97 | 21 | P < 0.001 | 21.6% | |
| cts-1/+ | +/+ | 49 | 46 | P > 0.05 | 93.9% | |
| cts-1/+ | cts-1/cts-1 | 118 | 140 | P > 0.05 | ||
| cts-1/cts-1 | cts-1/+ | 159 | 31 | P < 0.001 | ||
| +/+
|
cts-2/+
|
cts-2/cts-2
|
||||
| +/+ | cts-2/+ | 107 | 11 | P < 0.001 | 10.3% | |
| cts-2/+ | +/+ | 60 | 68 | P > 0.05 | 111.3% | |
| cts-2/+ | cts-2/cts-2 | 72 | 56 | P > 0.05 | ||
| cts-2/cts-2 | cts-2/+ | 151 | 15 | P < 0.001 | ||
Pollen Tube Growth Is Impaired in cts Mutants
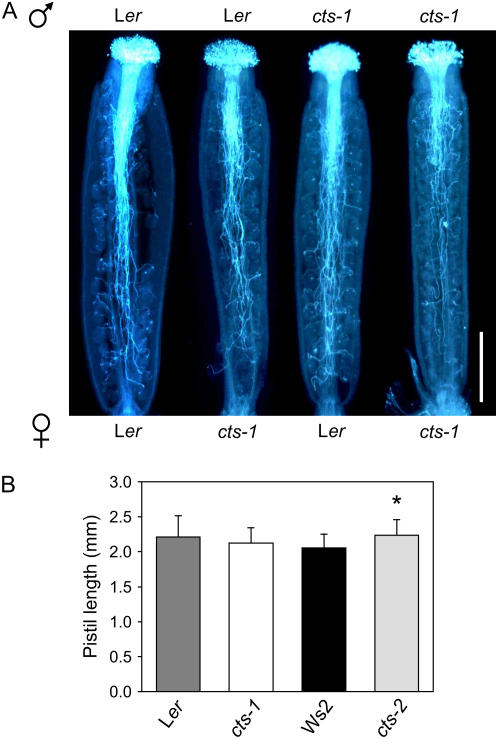
In cts mutants, unfertilized ovules appeared to predominate toward the base of the silique (Fig. 3C), suggesting a possible defect in pollen tube growth. Therefore, emasculated flowers of cts-1 and Ler were self- and cross-pollinated and pollen germination on the pistil allowed to proceed for 24 h. Subsequent aniline blue staining of callose in the pollen and pollen tube cell wall indicated that fewer cts-1 pollen tubes reached the base of cts-1 pistils when compared to Ler pollen inoculated onto both mutant and wild-type pistils. Furthermore, a higher proportion of cts-1 pollen tubes appeared to target ovules nearer to the stigmatal end of cts-1 pistils. In contrast, pollen tube growth appeared normal when cts-1 pollen was inoculated onto wild-type pistils (Fig. 4A), indicating that the presence of CTS in either male or female tissues was sufficient for wild-type pollen tube growth. Reduced pollen tube growth was also observed in selfed cts-2 compared to crosses in which one parent was wild type, Ws2 (data not shown). Pistils were of similar length in both wild types and mutants (Fig. 4B).
Figure 4.
Pollen tube growth in cts mutants and respective wild types. A, Pollen tube growth in mutant and wild-type pistils was determined in self- and cross-pollinated pistils. Pistils were removed 24 h after pollination and stained with aniline blue. Bar = 500 μm. B, Pistil lengths of Ler, cts-1, Ws2, and cts-2 flowers (stage 14). Values are means ± se (n = 22–28). Asterisk denotes that cts-2 is significantly different than Ws2 at 2%, as determined by ANOVA.
Peroxisomes Are Abundant in Many Floral Tissues
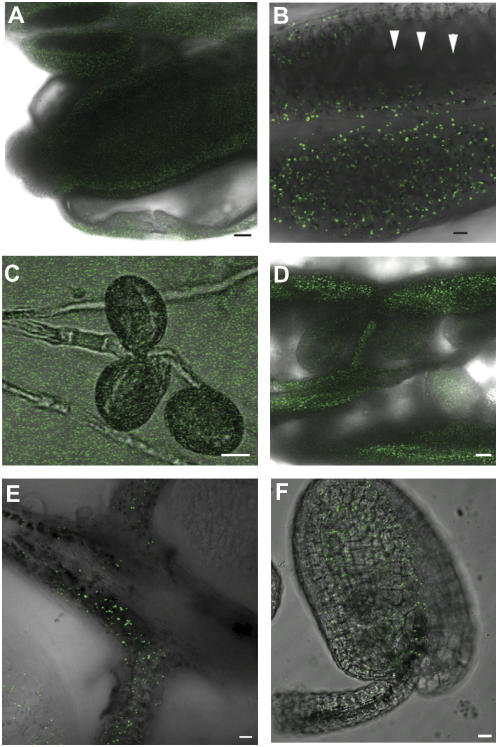
Because aniline blue staining indicated a role for CTS in both male and female tissues, we investigated the abundance of peroxisomes—the organelles that house the β-oxidation pathway—in different floral tissues. Peroxisomes were visualized by confocal laser-scanning microscopy in reproductive tissues from Arabidopsis plants expressing a peroxisomal-targeted GFP reporter (Cutler et al., 2000). Peroxisomes were abundant and readily detected in petal, sepal, pistil, and anther (Fig. 5, A and B; data not shown). However, expression of the GFP reporter was not detected in in vitro-germinated pollen (Fig. 5C), developing pollen grains within the anther locules (Fig. 5B), or pollen on the stigmatal surface (data not shown). Peroxisomes were abundant within the wall of the gynoecium (Fig. 5D) and within the transmitting tract tissue (Fig. 5, D and E). Peroxisomes were also present within the funiculus and fertilized ovules (Fig. 5F).
Figure 5.
Peroxisomes in reproductive tissues of Arabidopsis. Peroxisomes were visualized by confocal laser-scanning microscopy of plants expressing peroxisomally targeted enhanced GFP. A, Stage 12 flower showing GFP expression in petal, stamens (anther and filament), and pistil. Scale bar = 50 μm. B, Close-up of anther from stage 12 flower showing lack of expression in developing pollen grains (arrowheads) within the anther. Scale bar = 20 μm. C, Pollen germinated 4 h in vitro in Suc-containing medium, showing lack of expression. Scale bar = 10 μm. D, Dissected silique from stage 16 showing peroxisomes in the gynoecium wall and transmitting tissue. Scale bar = 50 μm. E, Higher magnification image showing peroxisomes within the transmitting tissue and funiculus. Scale bar = 10 μm. F, Peroxisomes in the fertilized ovule and funiculus from a stage 16 flower. Scale bar = 10 μm.
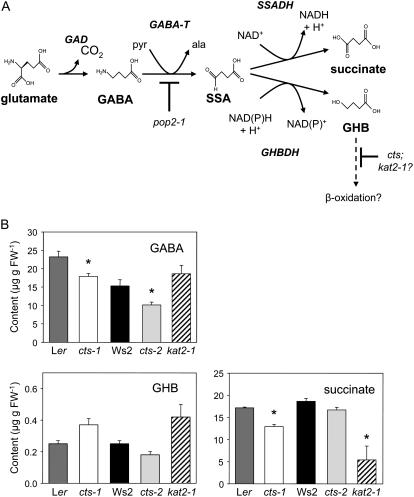
GABA Metabolism in Flowers of cts and kat2-1 Mutants
Because GABA is believed to be required for correct pollen tube growth and targeting (Palanivelu et al., 2003), we tested the hypothesis that CTS and, by extension, β-oxidation, might play a role in GABA catabolism, thereby assisting the generation of a GABA gradient in reproductive tissue, which permits optimal fertility. GABA and GABA shunt metabolites (Fig. 6A) were measured in flowers of both Ler and Ws wild types and in flowers of cts and kat2-1 mutants. Ws2 flowers contained significantly less GABA than those harvested from Ler plants (Fig. 6B). GABA content was significantly reduced in both cts-1 and cts-2, but not kat2-1, relative to wild type. Of the catabolites, γ-hydroxybutyrate (GHB) content appeared to be elevated in kat2-1, but levels were not significantly different in any of the genotypes tested. Succinate content was reduced in flowers of both cts-1 and kat2-1, but not in cts-2. Succinic semialdehyde, which is highly reactive, could not be detected, in agreement with a previous report (Palanivelu et al., 2003).
Figure 6.
GABA and GABA shunt metabolite content in flowers. A, Proposed scheme for GABA metabolism in Arabidopsis flowers. GABA is synthesized in the cytosol from Glu by Glu decarboxylase (GAD). The first step in GABA catabolism is catalyzed by mitochondrial GABA transaminase (GABA-T). In Arabidopsis flowers, this enzyme is encoded by POP2 and utilizes pyruvate (pyr) to yield Ala (ala) and succinic semialdehyde (SSA). Mutation of POP2 results in elevated GABA. In mitochondria, SSA undergoes oxidation to succinate, catalyzed by succinic semialdehyde dehydrogenase (SSADH). Alternatively, under hypoxia, SSA is reduced via the cytosolic enzyme, γ-hydroxybutyrate dehydrogenase (GHBDH; also known as SSA reductase), to yield GHB. It is possible that GHB, a short-chain hydroxy fatty acid, is metabolized further by β-oxidation, by analogy with mammalian systems (the dotted line indicates that this step is hypothetical). β-Oxidation of GHB is potentially inhibited in cts and kat2-1 mutants. B, Content of GABA, GHB, and succinate in Arabidopsis flowers. Values are means ± se (n = 4). A t test was performed comparing every mutant and its respective wild type (Ler or Ws2). Significant values (P < 0.05; critical t value 2.571) are indicated with an asterisk. Significant differences in GABA content were also found between the two Arabidopsis wild types.
A defect in the first step of GABA catabolism in the pop2-1 mutant leads to GABA hypersensitivity of pollen tube growth (Palanivelu et al., 2003; Fig. 6A). The GABA sensitivity of in vitro-grown cts and kat2-1 pollen tubes was therefore tested over a range of GABA concentrations from 0 to 1 mm, as described in Palanivelu et al. (2003), but was found to be highly variable (data not shown).
In Vitro Pollen Germination and Tube Growth Are Impaired in β-Oxidation Mutants in the Absence of Suc
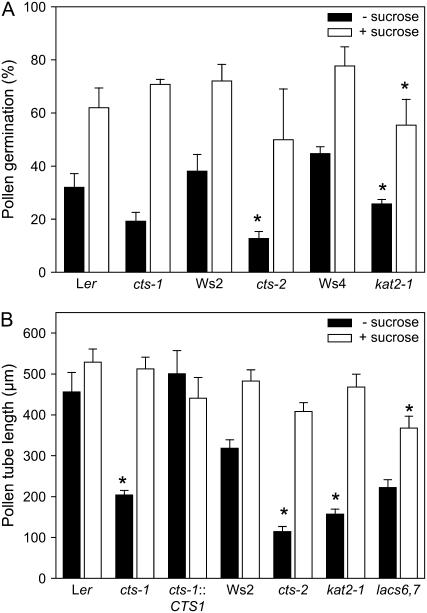
To investigate further the defect in the performance of cts mutant pollen, we measured pollen germination and tube growth in vitro. Pollen was germinated in isoosmotic medium containing 18% polyethylene glycol (PEG) or 16% PEG plus 2% Suc. The percentage of wild-type pollen germination varied between experiments, as has been reported previously for Arabidopsis (Johnson-Brousseau and McCormick, 2004); however, when pollen was germinated in the absence of an exogenous carbon source, we consistently observed a reduction in pollen germination (of between 40% and 65%) in cts and kat2-1 mutants (Fig. 7A). When Suc was included in the germination medium, the percentage germination increased in all genotypes and there was no significant difference between mutants and their respective wild types, with the exception of kat2-1. Similarly, we also investigated pollen tube growth in vitro in the presence and absence of a carbon source. Ler pollen tubes were of similar length in both media, but Ws2 pollen tubes tended to grow longer in the presence of Suc (Fig. 7B). In Suc medium, pollen tubes of the β-oxidation mutants, cts-1, cts-2, and kat2-1, were of similar length to those of the respective wild types. However, mutants produced significantly shorter pollen tubes in the absence of a carbon source. cts-1 plants complemented with the CTS genomic clone produced pollen tubes of wild-type length, confirming that loss of CTS function is responsible for the defect in pollen tube growth. Inclusion of auxin in the medium did not promote germination or tube growth of either mutant or wild-type pollen (data not shown).
Figure 7.
Pollen germination and tube growth in vitro. A, Pollen from stage 14 flowers was germinated in vitro in the presence or absence of a carbon source (2% Suc). Values are means ± se of three independent slides (approximately 800 grains/slide). B, Pollen tube growth in different genotypes: Ler (wild type), cts-1, and cts-1 transformed with bacterial artificial chromosome clone 159N1 (cts-1∷CTS); Ws2 (wild type), cts-2, kat2-1, and lacs6-1,lacs7-1. Pollen was germinated in vitro in the presence or absence of a carbon source (2% Suc) and pollen tube lengths of germinated pollen determined after 18 h. Values are means ± se (n = 50). Asterisks denote significant differences from wild type at 5%, as determined following ANOVA, using the lsds on the transformed data for A. Data presented in A and B are representative of several independent experiments.
DISCUSSION
cts Mutants Have a Subtle Vegetative and Floral Phenotype
Once cts plants had developed photosynthetic competence, the lack of CTS had only a minor effect on vegetative growth, with reduced leaf number and area in cts-1, but not cts-2 (Supplemental Fig. S1). This is in agreement with the phenotype reported for the pxa1 allele (Zolman et al., 2001) and the assertion that β-oxidation of fatty acids does not play a prominent role in vegetative growth of the unstressed plant, although it is likely to serve a housekeeping function in membrane lipid turnover (Graham and Eastmond, 2002). The loss of CTS function was more pronounced during reproductive development: Both cts mutants produced more flowers and also more siliques than wild types. This may represent a compensation mechanism, whereby reduced seed set in individual siliques is offset by increased total silique production. Of the known β-oxidation mutants, only abnormal inflorescence meristem 1 (aim1), which is deficient in a MFP, has a reported floral/fertility phenotype (Richmond and Bleecker, 1999). However, compared to aim1 mutants, which exhibit severe defects in floral development and are practically sterile, the cts mutant has a much more subtle reproductive phenotype. Unlike aim1 (Richmond and Bleecker, 1999), floral morphology was normal in cts mutants, although they exhibited delayed filament extension (Fig. 1B), a phenotype that is shared by plants lacking functional allene oxide synthase and COI1, which are required for JA biosynthesis and perception, respectively (Feys et al., 1994; Park et al., 2002; von Malek et al., 2002). However, application of JA did not rescue the anther extension phenotype of cts flowers (data not shown). Delayed filament extension has also been reported for mutants in which auxin transport or signaling is perturbed; for example, the filaments of the auxin response transcription factor mutants arf6-2 and arf8-3 were shorter than those of wild-type stage 12 flowers, although they elongated further as the flowers matured, as was the case for cts alleles (Nagpal et al., 2005). Accordingly, application of 10 μm NAA to cts flower buds resulted in wild-type filament extension (Fig. 1D). This result is consistent with a role for CTS in the conversion of IBA to IAA, although it is possible that exogenously applied auxin bypasses a requirement for CTS unrelated to auxin metabolism.
Fertilization Is Impaired in β-Oxidation Mutants
A noticeable feature of the cts mutants was the production of shorter siliques (Fig. 2), a phenotype that is often indicative of reduced fertilization, as, for example, in the JA biosynthetic mutant, opr3 (Stintzi and Browse, 2000), and mutants lacking the VANGUARD pectin methylesterase, which is required for pollen tube growth (Jiang et al., 2005). Dissection of siliques revealed that fertilization was reduced in both cts-1 and cts-2, with a more marked effect in the latter allele (Fig. 3, A and B). Fertilization was also compromised in kat2-1 and, to a lesser extent, in the lacs6-1,lacs7-1 double mutant (Fig. 3B), implying a general role for β-oxidation in fertilization. Although cts-1 was complemented by a CTS promoter-ORF construct (Fig. 3A), cts mutants were not rescued by exogenous application of JA or its precursors, 18:3 and OPDA, demonstrating that the fertilization phenotype does not result from JA deficiency. Application of auxin also failed to rescue the cts fertilization defect, which is perhaps not surprising, given that Arabidopsis flowers contain abundant IAA and IAA conjugates (Aloni et al., 2006) and that conversion from IBA may only account for a relatively small proportion of the free IAA pool (Bartel et al., 2001). The fertility phenotype of lacs6-1,lacs7-1 was intermediate between that of cts mutants and kat2-1, suggesting that full fertility requires β-oxidation of a substrate that is handled by additional or alternative acyl-activating enzymes.
Pollen Tube Germination and Growth Are Impaired in cts Mutants
Whereas CTS is expressed ubiquitously, it should be noted that transcripts are not expressed preferentially in either male or female gametophytic tissues or in specific sporophytic tissues (Becker et al., 2003; Honys and Twell, 2003, 2004; Wellmer et al., 2004; Yu et al., 2005). Thus, CTS could play a role in either or both sporophytic or gametophytic tissues. Genetic analysis was undertaken to determine whether the fertility phenotype of cts mutants arises from a gametophytic defect. The genotypes of progeny obtained from selfed heterozygotes deviated from the Mendelian 1:2:1 ratio, the low frequency of cts homozygotes observed among +/cts self-progeny indicating strongly reduced transmission of cts (Howden et al., 1998). In reciprocal cross experiments, only 21.6% of pollen carrying cts-1 successfully transmitted the mutation and this effect was more severe in cts-2 (transmission efficiency 10.3%) in agreement with the observation that cts-2 plants exhibited greater reduction in fertilization than cts-1 (Fig. 3). However, when the pistil was heterozygous for cts, mutant pollen tubes did not discriminate between wild-type and mutant eggs and female transmission efficiency of cts alleles was close to 100% (Table I), indicating that expression of CTS in the female gametophyte does not contribute to fertilization.
In accordance with a role for CTS in pollen, aniline blue staining revealed that in vivo pollen tube growth was impaired when cts-1 and cts-2 mutants were selfed (Fig. 4A). However, this defect was not observed when only one parent bore the cts mutation, suggesting that CTS can support pollen tube growth when expressed in either the pollen or the female tissue alone. Taken together with the genetic analysis, this not only suggests that expression of CTS in pollen is important for pollen tube growth, but also indicates that CTS has a function in female sporophytic tissue that can contribute to this process. The presence of peroxisomes within the transmitting tract is consistent with a role for CTS in this tissue (Fig. 5E). Abundant peroxisomes were also visualized in petals, sepals, funiculus, and ovules, but were not observed in pollen of 35S∷GFP-MFP2 plants (Fig. 5). This reflects the properties of the 35S promoter, which is known not to be active in Arabidopsis pollen (Wilkinson et al., 1997). It should be noted, however, that pollen does indeed contain abundant peroxisomes, as revealed by studies using the pollen-specific LAT52 promoter to drive expression of a peroxisomally targeted enhanced cyan fluorescent protein in lily (Prado et al., 2004) and the ACX1 promoter to drive expression of a peroxisomal enhanced yellow fluorescent protein in Arabidopsis (Schilmiller et al., 2007).
Given the numerous functions of β-oxidation in plants (Baker et al., 2006), CTS could play more than one role in fertilization, including synthesis of JA, synthesis of IAA, provision of energy and carbon skeletons via lipid catabolism, or processing of a signal that affects pollen tube growth and guidance. Therefore, the biochemical basis for impaired pollen germination and tube growth was investigated using pollen germinated in vitro. Neither pollen germination nor tube growth was promoted by auxin (data not shown), which is in agreement with the inability of auxin to restore fertilization in cts mutants. Pollen germination in the absence of exogenous Suc was impaired in cts and other β-oxidation mutants, although this was a moderate effect compared to the severe seed germination phenotype of cts alleles (Russell et al., 2000; Footitt et al., 2006). In the absence of an exogenous carbon source, pollen tubes of cts and other β-oxidation mutants were shorter than their respective wild types, but this difference was not apparent when Suc was included in the medium (Fig. 7). These findings are consistent with the suggestion that catabolism of stored lipids during pollen maturation could provide sugars to support pollen germination and tube growth.
Pollen tubes grow at a higher rate in vivo than in vitro (Johnson and Preuss, 2002), suggesting that nutrients and/or signals from the stylar tissue contribute to growth through the transmitting tissue (Lord, 2000; Lord and Russell, 2002). Pollen represents a symplastically isolated sink, which must take up sugars from the apoplast, and pollen grains and tubes consequently express several plasma membrane-bound transporters, which mediate uptake of sugars and other nutrients at different stages of growth and development (Stadler et al., 1999; Truernit et al., 1999; Schneidereit et al., 2003, 2005; Scholz-Starke et al., 2003; Bock et al., 2006). Interestingly, hap3, an Arabidopsis mutant with short pollen tubes, has been attributed to a T-DNA insertion in the SUC1 gene, which encodes a Suc transporter expressed specifically in male tissues (Stadler et al., 1999; Johnson et al., 2004). This implies that the female sporophytic tissue plays a role in supporting pollen tube growth energetically, as suggested by aniline blue staining (Fig. 4A). β-Oxidation of fatty acids could serve to provide Suc via the glyoxylate cycle and gluconeogenesis, as is the case in germinating oilseeds, or, alternatively, could provide acetyl equivalents for respiration.
There is some disagreement in the literature as to whether the glyoxylate cycle operates in growing pollen tubes, dependent on the species examined (Zhang et al., 1994; Mellema et al., 2002). However, examination of microarray data indicates that transcripts encoding the glyoxylate cycle enzymes isocitrate lyase and malate synthase are virtually absent from pollen and indeed also from other floral tissues in Arabidopsis (Zimmermann et al., 2004). Reporter fusions and reverse transcription-PCR studies also demonstrated lack of expression of malate synthase in floral tissue, whereas KAT (PED1/KAT2) is highly expressed (Charlton et al., 2005). In contrast, peroxisomal citrate synthase (CSY2 and CSY3) transcripts are expressed throughout the flower (Zimmermann et al., 2004), suggesting that lipids can be used as a source of carbon for respiration, as has been proposed for germinating sunflower (Helianthus annuus) seeds (Reymond et al., 1992). In this scenario, acetyl-CoA produced by β-oxidation is converted to citrate, which is exported from the peroxisome and participates in the citric acid cycle (Pracharoenwattana et al., 2005). Thus, products of fatty acid catabolism can pass from the peroxisome to the mitochondrion independently of the glyoxylate cycle, as is the case in Arabidopsis mutants, which lack isocitrate lyase (Eastmond et al., 2000).
An energetic role for CTS (and by extension β-oxidation) in female tissues is perhaps less obvious than in symplastically isolated pollen because it might be expected that sugars would be available from photosynthetic tissues of the flower. However, the ability of wild-type (but not cts) flowers to metabolize exogenously applied fatty acids suggests that maternal tissues are competent in lipid catabolism, and transcriptome data also support the assertion that flowers can potentially respire lipids. Moreover, the fact that the presence of CTS in either the male or the female tissues is sufficient for full fertility suggests that this transporter fulfills the same biochemical function in both pollen and the female sporophyte. It may be, however, that CTS plays an as-yet unknown role required for efficient pollen tube growth, which is unrelated to lipid catabolism.
β-Oxidation Is Not Essential for Correct Pollen Tube Guidance
Although the data presented in this manuscript support an energetic role for CTS in fertility, we did not rule out the possibility that β-oxidation might be involved in the production or removal of pollen tube guidance cues. Signals guiding the pollen tube to the female gametophyte are as yet relatively poorly characterized (Higashiyama et al., 2003), but it has been shown recently that a GABA transaminase encoded by POP2 is required for correct pollen tube growth and targeting (Palanivelu et al., 2003). Because the GABA catabolite GHB, a short-chain hydroxy fatty acid, is a substrate for mitochondrial β-oxidation in mammals (Draye and Vamecq, 1987), we tested the hypothesis that CTS, and by extension, peroxisomal β-oxidation, might play a role in GABA catabolism in flowers.
In the pop2 mutant, which lacks the first step of GABA catabolism, floral GABA levels are elevated approximately 100-fold (Palanivelu et al., 2003). Blocking metabolism of GHB by β-oxidation might therefore be predicted to increase GHB and possibly also GABA levels, thus perturbing the gradient, which is optimal for pollen tube growth and targeting. However, GHB levels did not show statistically significant differences in either cts or kat2-1 flowers relative to wild type (Fig. 6B). Although GABA levels were decreased in flowers of cts-1 and cts-2, the content of kat2-1 flowers was unchanged. This suggests that, although there may be a minor contribution of CTS to GABA metabolism, any alteration in GABA content is unlikely to account for the reduced fertility that we observed in cts and kat2-1 mutants. Moreover, unlike pop2, which is affected in both pollen tube growth and guidance, the effect of cts mutant alleles appears to be specific to pollen tube growth because we failed to detect any defective guidance in aniline blue-staining experiments (Fig. 4). The fact that a reduction, rather than an increase, in GABA was observed may reflect the complex posttranslational regulation of the pathway (Bouché and Fromm, 2004). Interestingly, succinate (the product of succinic semialdehyde dehydrogenase) was decreased in cts-1 and kat2-1 flowers. Succinate is produced by both the peroxisomal glyoxylate cycle and the mitochondrial citric acid cycle, but because the former pathway does not appear to operate in flowers (see above), reduced succinate in flowers of β-oxidation mutants most likely reflects the reduced flux of citrate from the peroxisome to the citric acid cycle.
CONCLUSION
The CTS ATP-binding cassette transporter plays a key role in regulating import of substrates into the peroxisome for β-oxidation. Although CTS was originally identified as a gene important for germination and seedling establishment, we show here that it is also required for full fertility in Arabidopsis. We have shown that CTS is required for efficient germination of pollen and that a defect in pollen tube growth is associated with reduced fertility of cts mutants, with CTS function in both male and female tissues contributing to pollen tube growth in vivo. We have tested three potential biochemical functions of CTS, synthesis of JA and IAA and catabolism of GABA, but did not find evidence that any of these functions underpin the fertilization phenotype of cts mutants. Although we cannot rule out a role for CTS (and β-oxidation) in processing an as-yet unidentified signaling molecule required for efficient fertilization, our data are consistent with the hypothesis that CTS contributes to fertilization via the provision of energy and carbon skeletons for the actively growing pollen tube.
MATERIALS AND METHODS
Plant Material
Isolation of cts-1 and cts-2 has been described previously (Footitt et al., 2002). Seeds of kat2-1 and lacs6-1,lacs7-1 were the kind gifts of Professor Steve Smith (University of Western Australia) and Dr. Martin Fulda (University of Göttingen), respectively.
Growth of Arabidopsis
Afterripened Arabidopsis (Arabidopsis thaliana) seeds of the mutants, cts-1, cts-2, and their respective wild types (Ler and Ws2), were germinated as described in Footitt et al. (2006). After 7 d, germinated seedlings were transplanted to soil and plants grown to maturity in controlled environment rooms (16-h light at 23°C and 70% relative humidity/8-h dark at 18°C and 80% relative humidity). During the light phase, the incident photosynthetically active radiation was 150 to 175 μmol m−2 s−1 at the soil level. The position of plant trays was rotated to minimize light effects.
Floral and Fertilization Phenotypes
Cumulative flowering on the primary inflorescence was measured daily from the onset of flowering. Each day, flowering buds were marked by applying acrylic paint to the pedicel. Flowering was defined as first appearance of petals from within the enclosing sepals (stage 13; Smyth et al., 1990). At floral stage 13, sepals and petals were removed to expose the anthers and pistil. The ratio of long stamen/pistil length was recorded for each mutant and wild type. Anthers were also removed from flowers at stage 13 and pollen viability determined using Alexander's stain (Alexander, 1969). The incidence of fertilization and abortion of ovules in siliques derived from the primary inflorescence was determined at 5, 10, and 15 d after flowering (DAF). Unfertilized ovules appear white, whereas aborted ovules are shrunken and dark in color. Silique size was measured at increasing DAF.
The ability of JA (2.0 mm; Sigma) and its precursors OPDA (3.4 mm; Larodan AB), and α-linolenic acid (3.3 mm; Nu-Chek Prep) to rescue floral and fertilization phenotypes was tested by painting floral buds on the primary inflorescence daily. Solutions (including controls) contained 0.01% (w/v) Tween 20 as a wetting agent. Similarly, auxins (NAA and IAA) were applied to flower buds by spraying with a 10 μm aqueous solution.
Complementation of cts-1
A plant transformation vector, pG0229-T, was generated by transferring the terminator region of pUC18-spGFP6 (M. Suter-Grotemeyer and D. Rentsch, unpublished data) to the vector pGreenII0229 (Hellens et al., 2000). First, annealed oligonucleotides (5′-CTAGAGGATCCGCATG-3′ and 5′-CGGATCCT-3′) were ligated into the XbaI/SphI sites of pUC18-spGFP6 to introduce a BamHI site. The spacer-GFP terminator cassette of pUC18-spGFP6 was excised with KpnI (made blunt with T4 DNA polymerase [New England Biolabs]) and SmaI and ligated into the NotI (blunt-ended)/SmaI sites of pGreenII0229. The spacer-GFP cassette was excised with BamHI and the plasmid religated to yield pG0229-T.
A CTS promoter-ORF cassette was prepared in several stages. A promoter fragment corresponding to 2,638 to 1,445 bp upstream of the ATG was amplified with primers CTS ProFW2 (5′-GAGTACTTGGAAGAAGGCGGTGA-3′) and CTS ProRV9 (5′-ATTGTACACCGCATGATTGAAGCACA-3′) and ligated into a blunted ApaI site of pBluescriptII SK− (Stratagene) to generate the plasmid, pSKPro-5′. A promoter fragment corresponding to 1,507 bp upstream and 38 bp downstream of the ATG was amplified with primers CTS ProFW4 (5′-GGAGTGATGTAATATGTACTTATCAGA-3′) and CTS ProRV (5′-CCGCGGCCCCGCTCAGTTAACTGCAATAG-3′; bold type indicates silent nucleotide changes to introduce the SacII site) and ligated into the SmaI site of pBluescriptII SK−, to yield pSKPro-3′. The ORF of CTS was then amplified in two parts using cloned cDNA as template. The 5′ fragment amplified by primer CTS5FW (5′-GGCCGCGGTCTTGTAGCGTCAAGACGGA-3′; bold type indicates silent nucleotide changes to introduce the SacII site) and CTS5RV (5′-GCCTTTGAATTAGTAGCAGATTCC-3′) and cloned in pCR-Blunt II Topo (Invitrogen) to yield pCRBlunt ORF5′, and the 3′ fragment amplified by primer CTS3FW2 (5′-GATCGGCAAAATGATGCGATGGT-3′) and CTS3RVwStop (5′-CCCGGGTCACTCTGTTGTCTGTTCGATCGA-3′; bold type indicates the introduced SmaI site) was restricted with PstI and ligated in the PstI/EcoRV sites of pBluescriptII SK−, to generate pSKORF3′. The promoter and the 5′ portion of the CTS ORF were assembled in a three-way ligation between pSK Pro-5′ restricted with BbsI/PstI, pSK Pro-3′ restricted with BbsI/SacII, and pCRBlunt ORF5′ restricted with SacII/PstI, to give pSK ProORF5′. The 3′ portion of the CTS ORF was excised from pSK ORF3′ with PstI/SmaI and cloned in the corresponding sites of pSK ProORF5′ to yield pSK ProORF. Finally, the promoter-ORF cassette was excised with KpnI/SmaI and ligated into the corresponding sites of pG0229-T. The construct pG0229-T/CTS prom-ORF was introduced into cts-1 plants by Agrobacterium-mediated transformation. Seeds of transformed plants were sown in soil and after the appearance of the first two true leaves sprayed repeatedly with a 150-mg/L solution of glufosinate ammonium (Bayer CropScience Limited) to select for transgenic plants.
Complementation of cts-1 with bacterial artificial chromosome clones was as described in Footitt et al. (2002).
Genetic Consequences of cts Mutation
Reciprocal crosses were performed between plants heterozygous for the cts-1 and cts-2 mutations and (1) wild-type plants or (2) homozygous mutant plants. Plants heterozygous for cts alleles were also allowed to self-fertilize. Mature siliques were collected and dried for 1 week prior to sterilization and plating on B5 agarose. Seeds were stratified at 4°C in the dark for 2 d and then transferred to germination conditions, as indicated in Footitt et al. (2006). cts-1/cts-1 and cts-2/cts-2 offspring were scored by failure to germinate under these conditions. Wild-type and heterozygous plants were scored by PCR. Genomic DNA was prepared from seedlings by the method of Edwards et al. (1991), modified for tissue disruption using a Tissue-Lyser (Qiagen Ltd.). PCR reactions contained 1× PCR buffer, 1 unit Taq polymerase (Promega), 1.5 mm MgCl2, 0.2 μm dNTPs, 10 pmol primers (see below). For Ws2/cts-2 progeny, cycle conditions were 94°C, 3 min; 35 cycles of 94°C, 30 s; 55°C, 45 s; 72°C, 3 min; and a final extension of 10 min at 72°C; primers were: DSF1 (5′-TCTAGCTAAGTGGTTGTTGTTGTTGTTAC-3′), DSR2 (5′-CATAGAATGCTATGCTTTCCGAATGAGTC-3′), and JL-202 T-DNA LB primer (5′-CATTTTATAATAACGCTGCGGACATCT-3′). For Ler/cts-1 progeny, cycle conditions were 94°C, 3 min; 32 cycles of 94°C, 30 s; 45°C, 45 s; 72°C, 2 min; and a final extension of 10 min at 72°C; primers were cts-cds-forward-04 (5′-GAGATCTTCTATGTGCCGCAACG-3′), cts-cds-reverse-05 (5′-CTTTTCACTGAATCAATTTCAGCATCC-3′), and ChrIVRBF (5′-CCTTCTTTCTTCTCTTCCCCATTTGGTC-3′). Transmission efficiencies were calculated as outlined in Howden et al. (1998) and the predicted frequency of cts mutants in self-progeny of +/cts heterozygotes was calculated from transmission ratios, as described in Park et al. (1998).
Pollen Tube Growth in Vivo
Pollen tube growth in mutant and wild-type pistils was determined in self- and cross-pollinated pistils. Twenty-four hours after pollination, pistils were removed and stained in aniline blue decolorized with activated charcoal (Muschietti et al., 1994). Images were recorded using a Zeiss Axiophot microscope (Karl Zeiss Ltd), a Leica DFC300FX digital camera, and IM50 Image Manager software (Leica Microsystems).
Pollen Germination and Tube Growth in Vitro
For each genotype, pollen from two flowers was cultured in suspended drops in either control medium [18% (w/v) PEG-3550, 1 mm CaCl2, 1 mm Ca(NO3)2, 1 mm MgSO4, 0.015% (w/v) boric acid, pH 6.5] or Suc medium (control medium with 16% [w/v] PEG-3550 and 2% [w/v] Suc). Control and Suc medium were isoosmotic as tested using a vapor pressure osmometer (Wescor). Pollen was incubated in a humid chamber for 16 h in hanging drops on microscope slides. Germination was scored by microscopic examination. Tubes of germinated pollen grains were visualized with a Zeiss Axiovert 135 inverted microscope (Karl Zeiss Ltd), and measured using QWIN image acquisition software (Leica Microsystems). The effect of GABA and GHB on pollen tube growth in vitro was determined as described in Palanivelu et al. (2003).
Statistical Treatments
ANOVA was used to analyze data comprising the percentage fertility, pistil length, percentage pollen germination, and pollen tube length. From the ANOVAs, the appropriate lsds at the 5% level of significance were used to compare means. A logit transformation was required for the percentage data, but no transformations to other data were required for these analyses.
Localization of Peroxisomes in Floral Tissue Using Confocal Microscopy
A line containing a 35S∷GFP-MFP2 fusion protein that is targeted to the peroxisome (Cutler et al., 2000) was used. Flowers of the indicated developmental stage were dissected and mounted in water and enhanced GFP fluorescence visualized on a Zeiss LSM 510 inverted confocal microscope equipped with 10×, 40×, and 63× oil immersion objectives. Excitation was with an argon laser at 488 nm and fluorescence detection using a 505- to 530-nm band-pass filter. Postacquisition image processing was done using LSM 5 browser software (Zeiss) and the Adobe Photoshop suite of programs.
Measurement of GABA and GHB
Plants were grown as described in “Plant Material.” Flowers (stage 13) were removed from primary inflorescences at the same time each day, over a 5-d period, and frozen in liquid nitrogen. Floral tissue was freeze dried prior to extraction and derivatization and metabolite content was determined by gas chromatography-mass spectrometry exactly as detailed in Roessner-Tunali et al. (2003), with the exception that retention time standards used were as described in Fait et al. (2006). In addition, GHB was added to the compounds that could be detected by this protocol by running an aliquot of chemically pure GHB purchased from Sigma-Aldrich.
Vegetative Phenotype
Plants of cts-1 and Ler were harvested commencing 10 d following transfer to soil and at 5-d intervals until siliques began to shatter. At each harvest, 10 plants were analyzed for rosette and cauline leaf number, area, and dry weight. Silique number and dry weight were also determined, as was the dry weight of the remainder of the aerial plant parts (stem and flowers). Dry weights were determined after 24 h at 90°C. Leaf areas were determined by analysis of leaf images using a Gel Doc 2000 with Quantity One software (Bio-Rad). All data are presented as the mean ± se. Cuticle integrity was tested in 7-d-old seedlings, as described in Tanaka et al. (2004). The cuticles of cts seedlings were indistinguishable from those of wild types (data not shown).
Photosynthesis and Chlorophyll Content
Chlorophyll was extracted from single rosette leaves of known fresh weight with 80% acetone. Chlorophyll content was determined spectrophotometrically after Hendry and Price (1993). Photosynthesis was measured by infrared gas analysis at a light intensity of 400 μmol m−2 s−1 at 20°C after Dutilleul et al. (2003).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Vegetative phenotype of cts mutants.
Supplemental Table S1. The observed and predicted frequency of cts mutants in self-progeny of +/cts heterozygotes.
Supplementary Material
Acknowledgments
Seeds of kat2-1 and lacs6-1,lacs7-1 were the kind gifts of Professor Steve Smith (University of Western Australia) and Dr. Martin Fulda (University of Göttingen), respectively. We thank Raffaella Carzaniga and Jean Devonshire of the Centre for Bioimaging (Rothamsted Research) for advice with microscopy, Simon Driscoll (Rothamsted Research) for his assistance with photosynthesis measurements, and Steve Powers (Rothamsted Research) for statistical advice. pUC18-spGFP6 was the generous gift of M. Suter-Grotemeyer and D. Rentsch (University of Berne).
This work was supported by the Biology and Biotechnology Research Council (grant nos. P19770 to A.B. and P19769 to F.L.T. and M.H.), by the Biology and Biotechnology Research Council, UK (grant-aided support to Rothamsted Research), and by the Minerva foundation for metabolite analysis performed at the Max Planck Institute (grant to A.F.).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Afitlhile MM, Fukushige H, Nishimura M, Hildebrand DF (2005) A defect in glyoxysomal fatty acid beta-oxidation reduces jasmonic acid accumulation in Arabidopsis. Plant Physiol Biochem 43 603–609 [DOI] [PubMed] [Google Scholar]
- Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44 117–122 [DOI] [PubMed] [Google Scholar]
- Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of auxin in regulating Arabidopsis flower development. Planta 223 315–328 [DOI] [PubMed] [Google Scholar]
- Baker A, Graham IA, Holdsworth M, Smith SJ, Theodoulou FL (2006) Chewing the fat: β-oxidation in signalling and development. Trends Plant Sci 11 124–132 [DOI] [PubMed] [Google Scholar]
- Baker HG, Baker I (1979) Starch in angiosperm pollen grains and its evolutionary significance. Am J Bot 66 591–600 [Google Scholar]
- Bartel B, LeClere S, Magidin M, Zolman BK (2001) Inputs to the active indole-3-acetic acid pool: de novo synthesis, conjugate hydrolysis, and indole-3-buytric acid beta-oxidation. J Plant Growth Regul 20 198–216 [Google Scholar]
- Becker JD, Boavida LC, Carneiro J, Haury M, Feijo JA (2003) Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiol 133 713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi KD, Twell D, Sze H (2006) Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol 140 1151–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9 110–115 [DOI] [PubMed] [Google Scholar]
- Charlton WL, Johnson B, Graham IA, Baker A (2005) Non-coordinate expression of peroxisome biogenesis, beta-oxidation and glyoxylate cycle genes in mature Arabidopsis plants. Plant Cell Rep 23 647–653 [DOI] [PubMed] [Google Scholar]
- Charzynska M, Murgia M, Cresti M (1989) Ultrastructure of the vegetative cell of Brassica napus pollen with particular reference to microbodies. Protoplasma 152 22–28 [Google Scholar]
- Cruz Castillo M, Martinez C, Buchala A, Metraux JP, Leon J (2004) Gene-specific involvement of beta-oxidation in wound-activated responses in Arabidopsis. Plant Physiol 135 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR (2000) Random GFP∷cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Imhoff V, Guern J (1996) Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198 532–541 [DOI] [PubMed] [Google Scholar]
- Draye JP, Vamecq J (1987) The inhibition by valproic acid of the mitochondrial oxidation of monocarboxylic and omega-hydroxymonocarboxylic acids: possible implications for the metabolism of gamma-aminobutyric acid. J Biochem (Tokyo) 102 235–242 [DOI] [PubMed] [Google Scholar]
- Dure LS III, Waters LC (1965) Long-lived messenger RNA: evidence from cotton seed germination. Science 147 410–412 [DOI] [PubMed] [Google Scholar]
- Dutilleul C, Driscoll S, Cornic G, De Paepe R, Foyer CH, Noctor G (2003) Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol 131 264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ (2006) SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ, Germain V, Lange PR, Bryce JH, Smith SM, Graham IA (2000) Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc Natl Acad Sci USA 97 5669–5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fait A, Angelovicic R, Less H, Ohad I, Urbanczyk-Wochniak E, Fernie AR, Galili G (2006) Arabidopsis seed development and germination is associated with temporally distinct metabolic shifts. Plant Physiol 142 839–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatime are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D (2000) Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 12 2001–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Marquez J, Schmuths H, Baker A, Theodoulou FL, Holdsworth M (2006) Analysis of the role of COMATOSE and peroxisomal beta-oxidation in the determination of germination potential in Arabidopsis. J Exp Bot 57 2805–2814 [DOI] [PubMed] [Google Scholar]
- Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson T, Graham I, Baker A, Holdsworth M (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J 21 2912–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda M, Schnurr J, Abbadi A, Heinz E, Browse J (2004) Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell 16 394–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain V, Rylott EL, Larson TR, Sherson SM, Bechtold N, Carde JP, Bryce JH, Graham IA, Smith SM (2001) Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J 28 1–12 [DOI] [PubMed] [Google Scholar]
- Graham IA, Eastmond PJ (2002) Pathways of straight and branched chain fatty acid catabolism in higher plants. Prog Lipid Res 41 156–181 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nito K, Takei-Hoshi R, Yagi M, Kondo M, Suenaga A, Yamaya T, Nishimura M (2002) Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid β-oxidation. Plant Cell Physiol 43 1–11 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42 819–832 [DOI] [PubMed] [Google Scholar]
- Hendry GAF, Price AH (1993) Stress indicators: chlorophylls and carotenoids. In GAF Hendry, JP Grime, eds, Methods in Comparative Plant Ecology, Chapman and Hall, London, pp 148–152
- Higashiyama T, Kuroiwa H, Kuroiwa T (2003) Pollen-tube guidance: beacons from the female gametophyte. Curr Opin Plant Biol 6 36–41 [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D (2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 132 640–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5 R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Park SK, Moore JM, Orme J, Grossniklaus U, Twell D (1998) Selection of T-DNA-tagged male and female gametophytic mutants by segregation distortion in Arabidopsis. Genetics 149 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh K, Huang AH (2005) Lipid-rich tapetosomes in Brassica tapetum are composed of oleosin-coated oil droplets and vesicles, both assembled in and then detached from the endoplasmic reticulum. Plant J 43 889–899 [DOI] [PubMed] [Google Scholar]
- Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D (2005) VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Preuss D (2002) Plotting a course: multiple signals guide pollen tubes to their targets. Dev Cell 2 273–281 [DOI] [PubMed] [Google Scholar]
- Johnson MA, von Besser K, Zhou Q, Smith E, Aux G, Patton D, Levin JZ, Preuss D (2004) Arabidopsis hapless mutations define essential gametophytic functions. Genetics 168 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Brousseau S, McCormick S (2004) A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically-expressed genes. Plant J 39 761–775 [DOI] [PubMed] [Google Scholar]
- Kim HU, Hsieh K, Ratnayake C, Huang AH (2002) A novel group of oleosins is present inside the pollen of Arabidopsis. J Biol Chem 277 22677–22684 [DOI] [PubMed] [Google Scholar]
- Kuang A, Musgrave ME (1996) Dynamics of vegetative cytoplasm during generative cell formation and pollen maturation in Arabidopsis thaliana. Protoplasma 194 81–90 [DOI] [PubMed] [Google Scholar]
- Li C, Schilmiller AL, Liu G, Lee GI, Jayanty S, Sageman C, Vrebalov J, Giovannoni JJ, Yagi K, Kobayashi Y, et al (2005) Role of beta-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell 17 971–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord E (2000) Adhesion and cell movement during pollination: cherchez la femme. Trends Plant Sci 5 368–373 [DOI] [PubMed] [Google Scholar]
- Lord EM (2003) Adhesion and guidance in compatible pollination. J Exp Bot 54 47–54 [DOI] [PubMed] [Google Scholar]
- Lord EM, Russell SD (2002) The mechanisms of pollination and fertilization in plants. Annu Rev Cell Dev Biol 18 81–105 [DOI] [PubMed] [Google Scholar]
- Mascarenhas JP (1993) Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Browse J (1996) The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S, Yang H (2005) Is there more than one way to attract a pollen tube? Trends Plant Sci 10 260–263 [DOI] [PubMed] [Google Scholar]
- Mellema S, Eichenberger W, Rawyler A, Suter M, Tadege M, Kuhlemeier C (2002) The ethanolic fermentation pathway supports respiration and lipid biosynthesis in tobacco pollen. Plant J 30 329–336 [DOI] [PubMed] [Google Scholar]
- Muschietti J, Dircks L, Vancanneyt G, McCormick S (1994) LAT52 protein is essential for tomato pollen development: pollen expressing antisense LAT52 RNA hydrates and germinates abnormally and cannot achieve fertilization. Plant J 6 321–338 [DOI] [PubMed] [Google Scholar]
- Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, Guilfoyle TJ, Hagen G, Alonso JM, Cohen JD, Farmer EE, et al (2005) Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132 4107–4118 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114 47–59 [DOI] [PubMed] [Google Scholar]
- Park J-H, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31 1–12 [DOI] [PubMed] [Google Scholar]
- Park SK, Howden R, Twell D (1998) The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125 3789–3799 [DOI] [PubMed] [Google Scholar]
- Park SK, Twell D (2001) Novel patterns of ectopic cell plate growth and lipid body distribution in the Arabidopsis gemini pollen1 mutant. Plant Physiol 126 899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinfield-Wells H, Rylott EL, Gilday AD, Graham S, Job K, Larson TR, Graham IA (2005) Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J 43 861–872 [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana I, Cornah JE, Smith SM (2005) Arabidopsis peroxisomal citrate synthase is required for fatty acid respiration and seed germination. Plant Cell 17 2037–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado AM, Porterfield DM, Feijo JA (2004) Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 131 2707–2714 [DOI] [PubMed] [Google Scholar]
- Preuss D, Lemieux B, Yen G, Davis RW (1993) A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev 7 974–985 [DOI] [PubMed] [Google Scholar]
- Reymond P, Spiteri A, Dieuaide M, Gerhardt B, Pradet A (1992) Peroxisomal β-oxidation of fatty acids and citrate formation by a particulate fraction from early germinating sunflower seeds. Plant Physiol Biochem 30 153–161 [Google Scholar]
- Richmond TA, Bleecker AB (1999) A defect in beta-oxidation causes abnormal inflorescence development in Arabidopsis. Plant Cell 11 1911–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Garcia MI, M'rani-Alaoui M, Fernandez MC (2003) Behavior of storage lipids during development and germination of olive (Olea europaea L.) pollen. Protoplasma 221 237–244 [DOI] [PubMed] [Google Scholar]
- Roessner-Tunali U, Heggemann B, Lytovchenko A, Carrari F, Bruedigam C, Granot D, Fernie AR (2003) Metabolic profiling of transgenic tomato plants overexpressing hexokinase reveals that the influence of hexose phosphorylation diminishes during fruit development. Plant Physiol 133 84–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L, Larner V, Kurup S, Bougourd S, Holdsworth M (2000) The Arabidopsis COMATOSE locus regulates germination potential. Development 127 3759–3767 [DOI] [PubMed] [Google Scholar]
- Sanders PS, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler E, Goldberg RB (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes and enzyme in the jasmonic acid synthesis pathway. Plant Cell 12 1041–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F, Schaller A, Stintzi A (2004) Biosynthesis and metabolism of jasmonates. J Plant Growth Regul 23 179–199 [Google Scholar]
- Schilmiller AL, Koo AJ, Howe GA (2007) Functional diversification of acyl-CoA oxidases in jasmonic acid biosynthesis and action. Plant Physiol 143 812–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidereit A, Scholz-Starke J, Buttner M (2003) Functional characterization and expression analyses of the glucose-specific AtSTP9 monosaccharide transporter in pollen of Arabidopsis. Plant Physiol 133 182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidereit A, Scholz-Starke J, Sauer N, Buttner M (2005) AtSTP11, a pollen tube-specific monosaccharide transporter in Arabidopsis. Planta 221 48–55 [DOI] [PubMed] [Google Scholar]
- Scholz-Starke J, Buttner M, Sauer N (2003) AtSTP6, a new pollen-specific H+-monosaccharide symporter from Arabidopsis. Plant Physiol 131 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey JM, Fulda MS, Browse J (2003) Arabidopsis contains a large superfamily of acyl-activating enzymes: phylogenetic and biochemical analysis reveals a new class of acyl-Coenzyme A synthetases. Plant Physiol 132 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey JM, Fulda MS, Browse JA (2002) Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol 129 1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Truernit E, Gahrtz M, Sauer N (1999) The AtSUC1 sucrose carrier may represent the osmotic driving force for anther dehiscence and pollen tube growth in Arabidopsis. Plant J 19 269–278 [DOI] [PubMed] [Google Scholar]
- Stintzi A, Browse J (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci USA 97 10625–10630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y (2004) A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J 37 139–146 [DOI] [PubMed] [Google Scholar]
- Theodoulou FL, Holdsworth M, Baker A (2006) Peroxisomal ABC transporters. FEBS Lett 580 1139–1155 [DOI] [PubMed] [Google Scholar]
- Theodoulou FL, Job K, Slocombe SP, Footitt S, Holdsworth M, Baker A, Larson TR, Graham IA (2005) Jasmonic acid levels are reduced in COMATOSE ABC transporter mutants: implications for transport of jasmonate precursors into peroxisomes. Plant Physiol 137 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Stadler R, Baier K, Sauer N (1999) A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J 17 191–201 [DOI] [PubMed] [Google Scholar]
- Van Aelst AC, Pierson ES, Van Went JL, Cresti M (1993) Ultrastructural changes of Arabidopsis thaliana pollen during final maturation and rehydration. Zygote 1 173–179 [DOI] [PubMed] [Google Scholar]
- von Malek B, van der Graff E, Schneitz K, Keller B (2002) The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 216 187–192 [DOI] [PubMed] [Google Scholar]
- Wellmer F, Riechmann JL, Alves-Ferreira M, Meyerowitz EM (2004) Genome-wide analysis of spatial gene expression in Arabidopsis flowers. Plant Cell 16 1314–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J, Twell D, Lindsay K (1997) Activities of CaMV 35S and nos promoters in pollen: implications for field release of transgenic plants. J Exp Bot 48 265–275 [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen-tube growth. Nature 392 818–821 [DOI] [PubMed] [Google Scholar]
- Yu HJ, Hogan P, Sundaresan V (2005) Analysis of the female gametophyte transcriptome of Arabidopsis by comparative expression profiling. Plant Physiol 139 1853–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Laudencia-Chingcuanco DL, Comai L, Li M, Harada JJ (1994) Isocitrate lyase and malate synthase genes from Brassica napus L. are active in pollen. Plant Physiol 104 857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkl GM, Zwiebel BI, Grier DG, Preuss D (1999) Pollen-stigma adhesion in Arabidopsis: a species-specific interaction mediated by lipophilic molecules in the pollen exine. Development 126 5431–5440 [DOI] [PubMed] [Google Scholar]
- Zolman B, Silva ID, Bartel B (2001) The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol 127 1266–1278 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.