Abstract
The SILENT INFORMATION REGULATOR2 (SIR2) family proteins are NAD+-dependent histone deacetylases. Sir2 is involved in chromatin silencing at the mating-type loci, rDNA, and telomeres in yeast and is associated with lifespan extension in yeast, worms, and flies, but also in a broader range of additional functions. In this work, we investigated the role of OsSRT1, one of the two SIR2-related genes found in rice (Oryza sativa). We show that OsSRT1 is a widely expressed nuclear protein with higher levels in rapidly dividing tissues. OsSRT1 RNA interference induced an increase of histone H3K9 (lysine-9 of H3) acetylation and a decrease of H3K9 dimethylation, leading to H2O2 production, DNA fragmentation, cell death, and lesions mimicking plant hypersensitive responses during incompatible interactions with pathogens, whereas overexpression of OsSRT1 enhanced tolerance to oxidative stress. Transcript microarray analysis revealed that the transcription of many transposons and retrotransposons in addition to genes related to hypersensitive response and/or programmed cell death was activated. Chromatin immunoprecipitation assays showed that OsSRT1 down-regulation induced histone H3K9 acetylation on the transposable elements and some of the hypersensitive response-related genes, suggesting that these genes may be among the primary targets of deacetylation regulated by OsSRT1. Our data together suggest that the rice SIR2-like gene is required for safeguard against genome instability and cell damage to ensure plant cell growth, but likely implicates different molecular mechanisms than yeast and animal homologs.
Histone acetylation involves the transfer of acetyl groups from acetyl-CoA to Lys residues of histones. Hyperacetylation of histones leads to relaxation of chromatin structure and is associated with transcriptional activation, whereas hypoacetylation of histones induces chromatin compaction and gene repression (Carrozza et al., 2003). Histone acetylation is catalyzed by histone acetyltransferases, whereas histone deacetylation is catalyzed by histone deacetylases (HDACs). Plant HDACs can be grouped into four subclasses. Three of them have primary homology to the three classes of HDACs (RDP3, HDA1, and SIR2) found in yeast and animal cells (Pandey et al., 2002). The fourth class of plant HDACs (known as the HD2 class) is found only in plants (Lusser et al., 1997; Pandey et al., 2002).
The SILENT INFORMATION REGULATOR2 (SIR2) family proteins, known also as sirtuins, are NAD+-dependent protein deacetylases. They contain a 200-amino acid domain (Pfam designation PF02146) conserved from bacteria to humans (Frye, 2000). Based on variations in this domain, the eukaryotic SIR2 proteins fall into four main classes (Frye, 2000). Yeast SIR2 belongs to class I of sirtuin genes and is involved in chromatin silencing, DNA repair, and chromosome fidelity during meiosis (for review, see Blander and Guarente, 2004). Deletion of yeast SIR2 leads to histone H3 and histone H4 hyperacetylation of subtelomeric regions, the mating-type loci, and the rDNA loci (Robyr et al., 2002). Sir2-related proteins have been implicated in mediating lifespan increases in yeast, worms, and flies, but also in a broader range of additional functions (for review, see Blander and Guarente, 2004; Haigis and Guarente, 2006).
Yeast has four additional Sir2 homologs, termed Hst1 to Hst4, in addition to the founding member. All of the yeast members belong to class I of the Sir2-related proteins (Frye, 2000). Mammalian cells have seven members of the SIR2 family (SIRT1–SIRT7), distributed into all four classes (Frye, 2000). Three of the mammalian members are localized in the nucleus; the remaining members are either cytoplasmic or mitochondrial localized (for review, see Haigis and Guarente, 2006).
Plant genomes seem to contain relatively fewer SIR2 homologs than the other eukaryotes. In Arabidopsis (Arabidopsis thaliana), only two SIR2 family gene sequences (named atSRT1 and atSRT2) have been identified. Phylogenetic analysis of identified plant SIR2 homologs shows that they belong to only two of the four classes of the family, classes that have only plant and animal members (Pandey et al., 2002; Fig. 1). So far no physiological function has been assigned to plant Sir2-related proteins. As there are fewer SIR2-related genes found in plant genomes, important questions arise, such as whether plant Sir2-related proteins conserve similar functions as yeast and animal homologs. In this work, we studied the function of a rice (Oryza sativa) SIR2-like gene, OsSRT1 (also called OsSIRT701; Pandey et al., 2002), by transgenic approaches. Our data show that OsSRT1 was preferentially expressed in rapidly dividing young tissues/organs and the protein was nuclear localized. Phenotypic and molecular analysis of RNA interference (RNAi) transgenic plants suggests that OsSRT1 is involved in H3K9 (Lys-9 of H3) deacetylation required for transcriptional repression of transposable elements and apoptosis-related genes. Our data suggest that OsSRT1 may have a function in the safeguard against genome instability and DNA damage to ensure plant cell growth.
Figure 1.
Neighbor-joining tree of SIR2-related proteins from eukaryotes. Abbreviations are as follows (in parentheses): Arabidopsis (at), Caenorhabditis elegans (ce), Drosophila melanogaster (dm), Homo sapiens (hs), rice (os), Saccharomyces cerevisiae (sc), Schizosaccharomyces pombe (sp), wheat (ta), and maize (zm). Four subclasses are indicated.
RESULTS
Rice Genome Contains Two SIR2-Related Genes
Sequence analysis of the rice genome revealed two SIR2-related genes, named OsSRT1 and OsSRT2. OsSRT1 and other plant SRT1 homologs are found in the same class (class IV), whereas OsSRT2 belongs to class II of the SIR2-related genes (Pandey et al., 2002; Fig. 1). There were no plant members found in class I and class III of the SIR2 family. Plant predicted SRT1 proteins showed relatively high conservation. Only the N-terminal parts of the plant proteins were conserved with the animal homologs (data not shown). Northern-blot analysis revealed that OsSRT1 was generally expressed in different tested rice tissues, but with higher transcript levels detected in tissues with high cell proliferation rates, such as buds, seedlings, and developing panicles (Fig. 2A). The animal members of class IV proteins, such as human HsSIRT6 and HsSIRT7, are nuclear localized. To detect the subcellular localization of OsSRT1, the coding region of the cDNA was fused to the GFP-coding sequence under the control of the maize (Zea mays) ubiquitin promoter and transiently transfected into onion (Allium cepa) cells. The fusion protein was localized in the nucleus (Fig. 2B).
Figure 2.
Expression profiles of OsSRT1. A, Northern hybridization detection of OsSRT1 mRNA in different rice tissues or developmental stages. Actin transcripts were detected as controls. B, Nuclear localization of the OsSRT1-GFP fusion. Top, Onion skin cells transfected with OsSRT1-GFP photographed under a confocal microscope at 488 nm (left) and merged with the transmission image (right). Bottom, GFP alone. Bars = 40 μm. [See online article for color version of this figure.]
Down-Regulation of OsSRT1 by RNAi Induced Programmed Cell Death in Rice
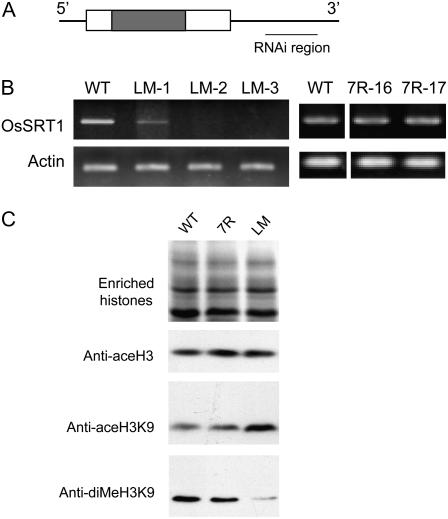
To study the physiological function of OsSRT1, a 412-bp segment of the 3′-untranslated region of the gene (Fig. 3A), which was not conserved with OsSRT2, was inserted in inverted repeats to build a construct for RNAi. The construct was used to transform an indica rice variety ‘Minghui63’. About 20 independent transgenic lines were produced and analyzed for OsSRT1 expression during the root regeneration stage. Three of them showed either reduced or no expression of the endogenous gene, suggesting an effect of RNAi (Fig. 3B). To further analyze whether there was any effect of OsSRT1 RNAi on histone modification, we did western-blot analyses using antibodies raised specifically against acetylated histone H3 and acetylated H3K9, because several nuclear SIR2 proteins in yeast and animal cells have been shown to be mainly involved in histone H3 and H3K9 deacetylation (Blander and Guarente, 2004). As H3K9 dimethylation is closely associated with H3K9 deacetylation (Strahl and Allis, 2000), we also tested with antibodies against dimethylated H3K9. As shown in Figure 3C, the OsSRT1 RNAi had little effect on overall histone H3 acetylation. However, the acetylation of H3K9 was induced, whereas the dimethylation of H3K9 was reduced, in agreement with the antagonistic relationship between H3K9 acetylation and dimethylation. Transgenic lines (7R-16 and 7R-17) with no reduction of OsSRT1 transcripts (Fig. 3B) showed no alteration in the histone modifications (Fig. 3C), suggesting that the phenotype was induced by OsSRT1 down-regulation.
Figure 3.
OsSRT1 RNAi affected overall H3K9 acetylation and dimethylation. A, Schematic representation of the OsSRT1 cDNA. The coding region is boxed. The dark region corresponds to the conserved catalytic domain. The DNA segment used to construct the RNAi vector is indicated. B, RT-PCR analysis of OsSRT1 transcripts in the wild type and three transgenic lines (LM-1–LM-3). A comparison between the wild type and two nonphenotypic transgenic lines (7R-16 and 7R-17) is shown on the right. Actin transcripts were detected as controls. C, Western-blot analysis of enriched histone fractions isolated from the wild type and pooled transgenic 7R or LM lines shown in B with antibodies against acetylated histone H3, acetylated H3K9, and dimethylated H3K9, as indicated. WT, Wild type.
The RNAi lines were selected for phenotype observation and further analysis. The RNAi plants at the two-leaf stage (about 14 d after germination) began to produce brown dots on leaves, which became larger at latter stages, leading to precocious leaf senescence (Fig. 4). Only two of the three RNAi lines could produce seeds. The severest line died before getting into maturity. The transgenic lines showing no alteration of OsSRT1 expression or histone modification did not manifest the phenotype (data not shown), suggesting that the lesion mimic phenotype was induced by OsSRT1 down-regulation. The lesions were reminiscent of cell death induced by hypersensitive responses during plant pathogen infections, suggesting that OsSRT1 RNAi might have induced programmed cell death (PCD). To test this hypothesis, young leaf sheaths (T1 generation) were incubated with 3,3′-diaminobenzidine (DAB) to detect H2O2 (Thordal-Christensen et al., 1997). H2O2 production was detected in cells of OsSRT1 RNAi leaf sheaths at day 7 after germination (before appearance of the symptom). At day 21 after germination, more cells produced H2O2 at higher levels (Fig. 5A). H2O2 production is an indicator of plant cells undergoing hypersensitive PCD during incompatible plant-pathogen interactions (Brodersen et al., 2002). Therefore, the lesion mimic phenotype of OsSRT1 RNAi plants suggested that the down-regulation of OsSRT1 induced PCD in rice. To confirm whether OsSRT1 RNAi induced PCD, the second leaves of 2-week-old wild-type and OsSRT1 RNAi (T1 generation) plants were fixed, sectioned, and processed for terminal deoxyribonucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL). TUNEL can sensitively detect DNA fragmentation, one of the hallmarks of PCD, by labeling exposed 3′ hydroxyl ends of DNA fragments using fluorescein-dUTP. The same leaf sections were simultaneously stained with propidium iodide to reveal all nuclei (red) in each section. None of the nuclei in wild type was TUNEL positive but, in contrast, most nuclei in the OsSRT1 RNAi leaf section were TUNEL positive (green; Fig. 5B), indicating that DNA damage was generally induced in the OsSRT1 RNAi leaves.
Figure 4.
OsSRT1 RNAi induced a lesion mimic phenotype. A, An OsSRT1 RNAi seedling at two-leaf stage (right) compared to a wild-type plant at the same age (left), showing that the development of lesions affected the growth. Bar = 3 cm. B, Enlarged view of the RNAi plants in A. Bar = 0.6 cm. C, An OsSRT1 RNAi plant at three-leaf stage (right) compared to a wild-type plant at the same age (left). Bar = 3 cm. D, Enlarged view of a leaf from the RNAi plant shown in C. Bar = 0.5 cm. E, Comparison of an RNAi plant (right) with the wild type (left) at tillering stage. Bar = 16 cm. F, Comparison of an RNAi plant (right) with the wild type (left) at mature stage. Bar = 20 cm. G, Enlarged views of leaves of the RNAi plant at tillering stages. Bar = 3 cm.
Figure 5.
OsSRT1 RNAi induced H2O2 production and genomic DNA fragmentation. A, DAB detection of H2O2 in OsSRT1 RNAi (a–d) and wild-type (e–h) plants at day 7 (a, e, c, and g) and day 21 (b, f, d, and h) after germination. Sections from leaf blades (a, b, e, and f) and from sheaths (c, d, g, and h) are shown. Bar = 20 μm. B, Detection of nuclear DNA fragmentation by in situ TUNEL assay. In situ TUNEL assay for detection of DNA cleavage was performed using young leaf tissues at day 21 after germination. The upper fifth of the leaf was used for assay. All the cross sections were counter-stained with propidium iodide. a and c are the corresponding negative controls for b and d, respectively. a and b are the leaves of the wild type, and c and d are the leaves of OsSRT1 RNAi plants. Bar = 28 μm.
Overexpression of OsSRT1 Enhanced Tolerance to Oxidative Stress
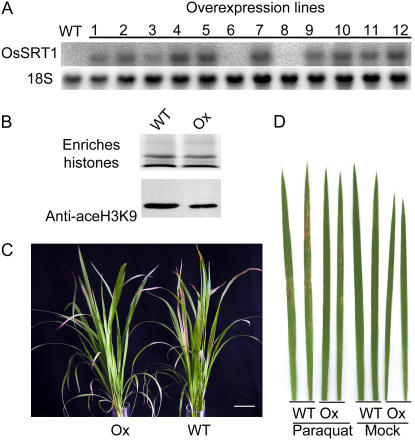
To further study the function of OsSRT1, the cDNA was inserted in an overexpression vector under the control of the maize ubiquitin promoter. More than 30 independent lines were obtained. Northern-blot analysis showed that most of the transgenic plants overexpressed OsSRT1 (Fig. 6A). Western-blot analysis of enriched histone fractions detected a decrease of H3K9 acetylation in overexpression plants (Fig. 6B). The overexpression plants showed no particular visible or morphological phenotype. However, when treated with paraquat (1,1′-dimethyl-4,4′-bipyridylium), an herbicide that induces oxidative stresses in plants, the overexpression plants showed an enhanced tolerance compared to the wild type, as demonstrated by fewer and smaller lesions observed on the overexpression plants than the wild type (Fig. 6, C and D), whereas no clear difference was seen between wild-type and transgenic siblings without overexpression of OsSRT1 (Supplemental Fig. S1). This suggests that the increased tolerance to paraquat was induced by OsSRT1 overexpression.
Figure 6.
Overexpression of OsSRT1 conferred tolerance to paraquat treatment. A, Northern-blot analysis of OsSRT1 overexpression in different transgenic lines compared to the wild type. The 18S ribosomal RNA levels were revealed as controls. B, Western-blot analysis of enriched histone fractions from pooled samples of the overexpression plants with antibodies against acetylated histone H3K9. C, Comparison of overexpression plants with wild-type ones challenged by 10 μm paraquat. D, Comparison of leaves from overexpression and wild type treated with or without 10 μm paraquat. WT, Wild type; Ox, overexpression plants. [See online article for color version of this figure.]
Transcriptomic Analysis Revealed Activation of Many Transposon and PCD-Related Genes
To study whether the down-regulation of OsSRT1 affected gene expression, we compared the transcripts of the RNAi to the wild-type plants by microarray analysis (Affymetrix). RNAs were isolated from young leaves of 11-d-old plants (before appearance of lesions in the RNAi plants). Analysis of data from three biological repeats revealed that 521 genes were up-regulated and 213 down-regulated (with q value at 5%). (The data are under the accession no. GSE7197 at http://www.ncbi.nlm.nih.gov/geo.) Gene ontology classification of the deregulated genes revealed that most categories had more up- than down-regulated genes, in agreement with the global up/down ratio (Table I). For instance, the transposon categories (both DNA and retroelements) had 40 members activated in the RNAi leaves, but only four DNA elements were repressed. Most of the activated transposons listed in Table II were not expressed in wild-type rice as revealed by background signals of the microarray hybridizations. In addition, a relatively large number of stress-responsive and stress-related (i.e. phenylpropanoid metabolism, defense response, DNA repair) genes were deregulated (Table I). To confirm the microarray data, we performed semiquantitative reverse transcription (RT)-PCR analysis of RNA isolated from OsSRT1 RNAi young leaves harvested at 7, 11, and 21 d after germination to compare with wild-type and OsSRT1 overexpression plants. As shown in Figure 7A, the expression of tested DNA and retroelements was induced early in 7-d-old RNAi leaves. Overexpression of OsSRT1 had a negative effect on the expression of the DNA elements, but seemed to have some positive effect on the two tested retroelements (Fig. 7A). It was not known at this stage whether the induction of the retroelement was directly related to the OsSRT1 overexpression or a consequence of an indirect effect induced by the overexpression.
Table I.
Gene ontology categories of up-regulated (Up) and down-regulated (Down) genes found by Affymetrix microarray analysis of OsSRT1 RNAi plants compared to the wild type
| Gene Ontology Nos. | Gene Ontology Annotation | Down | Up |
|---|---|---|---|
| GO:0003700 | Transcription factor activity | 16 | 30 |
| GO:0006281 | DNA repair | 0 | 2 |
| GO:0006313 | DNA transposition | 4 | 22 |
| GO:0032197 | Transposition, RNA mediated | 0 | 18 |
| GO:0012501 | PCD | 2 | 7 |
| GO:0045454 | Cell homeostasis | 0 | 5 |
| GO:0007165 | Signal transduction | 23 | 27 |
| GO:0006629 | Lipid metabolism | 21 | 23 |
| GO:0006810 | Transport | 28 | 53 |
| GO:0009698 | Phenylpropanoid metabolism | 5 | 18 |
| GO:0015979 | Photosynthesis | 2 | 1 |
| GO:0019538 | Protein metabolism | 35 | 62 |
| GO:0007049 | Cell cycle | 0 | 7 |
| GO:0005975 | Carbohydrate metabolism | 7 | 13 |
| GO:0006952 | Defense response | 24 | 41 |
| GO:0016043 | Cell organization and biogenesis | 25 | 50 |
| GO:0006139 | Nucleobase, nucleoside, nucleotide, and nucleic acid metabolism | 21 | 60 |
| GO:0007275 | Development | 24 | 60 |
| GO:0006519 | Amino acid and derivative metabolism | 10 | 35 |
| GO:0006950 | Response to stress | 44 | 84 |
| GO:0040007 | Growth | 11 | 14 |
| GO:0007568 | Aging | 5 | 2 |
| Unknown | Unknown | 143 | 275 |
| Total | 313 | 521 |
Table II.
Transposon elements induced by OsSRT1 RNAi
| Transposon Class | Family | Locus | Affymetrix Probe Sets | Log2 |
|---|---|---|---|---|
| Class I (retrotransposons) | Ty3-gypsy subclass retrotransposon family | Os03g19600 | Os.8570.3.S1_s_at | 4 |
| Os03g50670 | Os.48948.1.S1_x_at | 5.6 | ||
| Os02g32880 | Os.46692.1.S1_at | 5.4 | ||
| Os09g31930 | Os.52004.1.S1_at | 2.8 | ||
| Os03g50670 | Os.48948.1.S1_x_at | 5.6 | ||
| Os04g07770 | Os.41695.3.S1_x_at | 1.9 | ||
| Os10g08340 | OsAffx.8464.1.S1_at | 3.6 | ||
| Ty1-copia subclass retrotransposon family | Os08g03880 | Os.23300.1.S1_at | 4 | |
| Non-LTR retrotransposon family (LINE) | Os12g41440 | Os.20851.1.A1_x_at | 2.9 | |
| Os05g26730 | Os.20851.1.A1_x_at | 2.9 | ||
| Unclassified | Os04g48650 | Os.20851.1.A1_x_at | 2.9 | |
| Os05g26740 | Os.20851.1.A1_x_at | 2.9 | ||
| Os08g22520 | Os.20851.1.A1_x_at | 2.9 | ||
| Os03g33160 | Os.20851.1.A1_x_at | 2.9 | ||
| Os07g09760 | OsAffx.8923.1.S1_at | 2.5 | ||
| Os05g30290 | OsAffx.8923.1.S1_at | 2.5 | ||
| Os04g51870 | OsAffx.8923.1.S1_at | 2.5 | ||
| Os03g59190 | OsAffx.8923.1.S1_at | 2.5 | ||
| Os11g36110 | OsAffx.8923.1.S1_at | 2.5 | ||
| Os11g29950 | OsAffx.8923.1.S1_at | 2.5 | ||
| Os07g05440 | Os.50940.1.S1_a_at | 5.3 | ||
| Os08g10250 | Os.52458.1.S2_at | 1.6 | ||
| Os03g18880 | Os.53278.1.S1_at | 4.5 | ||
| Os02g14210 | OsAffx.24244.1.S1_at | 3.9 | ||
| Os08g35020 | Os.50495.1.S1_at | 5.5 | ||
| Os07g44720 | Os.51357.1.S1_at | 1.4 | ||
| Os07g31690 | Os.51357.1.S1_at | 1.4 | ||
| Os07g23980 | Os.26542.1.A1_at | 1.7 | ||
| Os07g20260 | Os.26542.1.A1_at | 1.7 | ||
| Class II (DNA transposons) | hAT-like transposase family | Os03g36550 | Os.23305.1.A1_at | 6 |
| Os06g11830 | Os.20614.3.S1_x_at | 9 | ||
| Os07g15340 | Os.20614.3.S1_x_at | 9 | ||
| Os11g40360 | OsAffx.424.1.S1_at | 8.4 | ||
| Os07g47800 | OsAffx.424.1.S1_at | 8.4 | ||
| Os10g02910 | OsAffx.424.1.S1_at | 8.4 | ||
| Pong subclass transposase family | Os10g31120 | OsAffx.8070.1.S1_x_at | 5.3 | |
| Os10g41350 | OsAffx.8070.1.S1_x_at | 5.3 | ||
| Os01g60590 | OsAffx.8070.1.S1_x_at | 5.3 | ||
| Os01g65240 | Os.31886.1.S1_at | 2.8 | ||
| Os01g60580 | OsAffx.21778.2.S1_at | 5.9 | ||
| Os01g27580 | OsAffx.8898.1.S1_at | 2.1 | ||
| Os12g12500 | OsAffx.8898.1.S1_at | 2.1 | ||
| Os05g43630 | OsAffx.8898.1.S1_at | 2.1 | ||
| CACTA, En/Spm subclass transposase family | Os01g60530 | Os.8248.2.S1_x_at | 7 | |
| Os10g30670 | Os.47371.1.S1_at | 5.4 | ||
| Os10g26910 | Os.48948.1.S1_x_at | 4.1 | ||
| Os10g21980 | Os.46692.1.S1_at | 7.6 | ||
| Os04g29620 | Os.46692.1.S1_at | 7.6 | ||
| Os11g22240 | Os.46692.1.S1_at | 7.6 | ||
| Os07g24940 | Os.6645.1.S1_s_at | 3.4 | ||
| Os07g24960 | Os.6645.1.S1_s_at | 3.4 | ||
| Os08g34390 | Os.56875.1.S1_at | 4.4 | ||
| Os07g32710 | OsAffx.22999.1.S1_at | 3.1 | ||
| Os10g26910 | OsAffx.26155.1.S1_x_at | 3.7 | ||
| Mutator subclass transposase family | Os08g34770 | Os.38884.2.S1_x_at | 4.6 | |
| Os03g28930 | Os.38884.2.S1_x_at | 4.6 | ||
| Os12g35100 | Os.38884.2.S1_x_at | 4.6 | ||
| Os09g03180 | Os.41695.3.S1_x_at | 1.9 | ||
| Os02g03240 | Os.41695.3.S1_x_at | 1.9 | ||
| Os05g07920 | Os.41695.3.S1_x_at | 1.9 | ||
| Os04g23470 | Os.41695.3.S1_x_at | 1.9 | ||
| Os10g26360 | Os.41695.3.S1_x_at | 1.9 | ||
| Os03g18060 | Os.41695.3.S1_x_at | 1.9 | ||
| Os03g47130 | Os.41695.3.S1_x_at | 1.9 | ||
| Os07g46020 | Os.41695.3.S1_x_at | 1.9 | ||
| Os04g12520 | Os.41695.3.S1_x_at | 1.9 | ||
| Os05g25470 | Os.41695.3.S1_x_at | 1.9 | ||
| Os06g29050 | Os.41695.3.S1_x_at | 1.9 | ||
| Os05g36060 | Os.41695.3.S1_x_at | 1.9 | ||
| Os08g28990 | Os.41695.3.S1_x_at | 1.9 | ||
| Os06g08070 | Os.41695.3.S1_x_at | 1.9 | ||
| Os01g18470 | Os.41695.3.S1_x_at | 1.9 | ||
| Os12g29530 | Os.41695.3.S1_x_at | 1.9 | ||
| Os01g28740 | Os.41695.3.S1_x_at | 1.9 | ||
| Os07g41430 | Os.41695.3.S1_x_at | 1.9 | ||
| MuDR transposase family | Os03g46080 | Os.41695.3.S1_x_at | 1.9 | |
| Os05g05090 | Os.41695.3.S1_x_at | 1.9 | ||
| Os11g31830 | Os.41695.3.S1_x_at | 1.9 | ||
| Os08g19970 | Os.41695.3.S1_x_at | 1.9 | ||
| Os12g08080 | Os.41695.3.S1_x_at | 1.9 | ||
| Unclassified | Os10g42160 | Os.48027.1.S1_x_at | 3 | |
| Os09g31940 | Os.52004.1.S1_at | 3.3 | ||
| Os12g32140 | OsAffx.14528.2.S1_x_at | 2.2 | ||
| Os05g03800 | OsAffx.14528.2.S1_x_at | 2.2 | ||
| Os06g47420 | Os.5212.2.S1_at | 1.9 | ||
| Os02g43370 | OsAffx.2947.1.S1_at | 1.6 | ||
| Os12g42190 | Os.19899.2.S1_at | 1.6 |
Figure 7.
Semiquantitative RT-PCR analysis of transposable elements (A) and PCD-related genes (B) of RNAs isolated from wild type (WT), OsSRT1 RNAi (LM-1, -2), or overexpression (OX-1, -2) plants at days 7, 11, and 21 after germination, respectively.
We compared the expression of two hypersensitive response (HSR201 and HSR203J) marker genes (Czernic et al., 1996) and a cytochrome P450 gene (called APO) that is closely related to wheat (Triticum aestivum) CYP709C1 and CYP709C3v2, both of which are suggested to be involved in wheat defense to pathogens (Kandel et al., 2005; Kong et al., 2005). HSR201 and APO, but not HSR203J, were found to be induced by OsSRT1 RNAi in the microarray data (Table III). Consistently, the RT-PCR results showed that HSR201 and APO, but not HSR203J, were activated early in 7-d-old RNAi plants (Fig. 7B). In contrast, HSR203J was repressed by OsSRT1 overexpression (Fig. 7B).
Table III.
Expression changes of apoptosis- and defense-related affected genes
| PCD-Related Genes | Locus | Function | Log2-Fold |
|---|---|---|---|
| HSR203J | Os05g33940 | Hypersensitive-related proteins | −0.3 |
| HSR201 | Os12g27254 | Hypersensitive-related proteins that are induced during bacterial infections of plant tissues | 3 |
| APO | Os03g037140 | Apoptosis; defense-related cytochrome P450 | 3.3 |
| OsSAG12 | Os09g32230 | Senescence-specific Cys protease | 3.4 |
| OsSAG13 | Os03g16230 | Senescence-associated Gene13 | 0 |
| PR1a | Os07g03690 | PR protein | 0.7 |
| PR5 | Os12g38150 | PR protein | 0.6 |
| PR8 | Os10g28080 | PR protein | 0.7 |
| PR10 | Os03g18850 | PR protein | 2.3 |
The microarray data revealed that the OsSRT1 RNAi induced SAG12 but not SAG13 (Table III), both of which are senescence-associated genes (Pontier et al., 1999; Brodersen et al., 2002). RT-PCR revealed that SAG12 was induced 11 d after germination. OsSRT1 overexpression repressed the SAG12 at day 21 after germination. The expression of SAG13 was not significantly altered by the deregulation of OsSRT1 (Fig. 7B). The microarray data also showed moderate induction of PATHOGENESIS-RELATED (PR) genes that are activated during hypersensitive responses (Table III). The RT-PCR results showed the induction of the tested PR genes 11 d after germination, later than that of HSR201 and APO (Fig. 7B), in agreement with the fact that PR gene induction is a downstream event of hypersensitive responses. OsSRT1 overexpression showed induction of PR1a and PR1b at day 7 after germination, but repression of the tested PR genes at day 21 after germination. These observations suggested that PR genes might be not the direct targets of OsSRT1 and that the deregulation of OsSRT1 might have an indirect impact on PR gene expression.
OsSRT1 RNAi Induced Histone H3K9 Acetylation on Transposable Elements
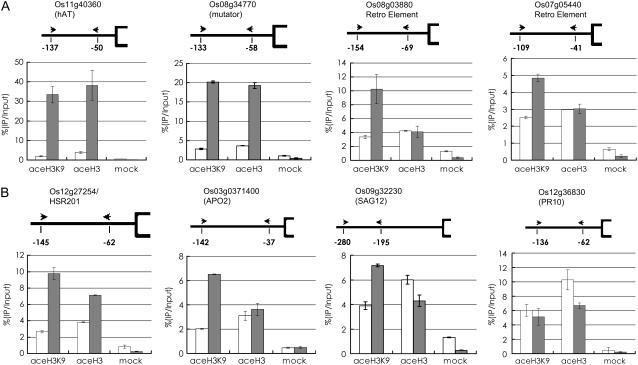
To study whether the activation of the transposable elements and PCD marker gene expression by down-regulation of OsSRT1 was linked to alterations in histone acetylation, we performed chromatin immunoprecipitation (ChIP) assays. Chromatin fragments isolated from 11-d-old leaves of wild-type and OsSRT1 RNAi plants were immunoprecipitated with antibodies against acetylated histone H3 or acetylated histone H3K9. The precipitated chromatin DNA was analyzed by real-time PCR to test for enrichment relative to nonprecipitated (input) genomic DNA. The enrichment of promoter fragments relative to input chromatin DNA in the wild type (arbitrarily assessed as 100%) was compared to that found for the transgenic plants. As shown in Figure 8, H3K9 acetylation was induced on both the tested DNA and retroelements. H3K9 acetylation was clearly induced on HSR201 and APO, in agreement with the expression data. In addition, the overall H3 acetylation was significantly induced on the DNA elements and HSR201. This suggested an induction of acetylation of other H3 Lys residues by H3K9 acetylation in the chromatin regions, as there is an agonistic relationship between different Lys residues for acetylation (Strahl and Allis, 2000). The increase of acetylation on SAG12 could also be observed, but to a lesser extent. No increase of H3K9 acetylation was observed on PR10, supporting the above-proposed hypothesis that the PR genes might not be direct targets of OsSRT1. However, transposons and retroelements, as well as some PCD marker genes (HSR201 and APO), might be among the primary targets of deacetylation induced by OsSRT1.
Figure 8.
Effect of OsSRT1 RNAi on histone H3 and H3K9 acetylation on transposable elements (A) and PCD-related genes (B). Nuclei were extracted from cross-linked rice seedlings, sonicated, and immunoprecipitated with antibodies specific to acetylated histone H3 (aceH3), acetylated H3K9 (aceH3K9), or without antibody (mock). The immunoprecipitates were analyzed by real-time PCR. The primer sets (arrowheads) are numbered for each gene, and the positions of the primers relative to the initiation ATG codon are indicated. The relative amounts of the PCR products compared to input chromatin from wild-type extracts (arbitrarily given as 100) are shown below the genes. Gray bars, OsSRT1 RNAi; white bars, wild type. Small bars represent sd from at least three repetitions.
DISCUSSION
Compared to other eukaryotes, plants have relatively fewer SIR2-related genes. This would suggest that the plant members may have a larger spectrum of functions compared to their yeast or animal counterparts. For instance, human SIRT1, SIRT6, and SIRT7 are localized to nucleus, but SIRT1 has been shown to regulate nonhistone proteins involved in apoptosis, cell survival, transcription, and metabolism. Our data showed that OsSRT1 is nuclear localized, suggesting that the rice protein may function mainly in the nucleus. Alternatively, the smaller number of SIR2-related genes found in plants may be compensated by other plant HDAC genes, as plants possess an additional class of HDAC genes, namely, HD2 (Lusser et al., 1997; Pandey et al., 2002). This plant-specific class of HDACs lacks sequence similarity with other classes of HDACs that are conserved from plants to humans (Pandey et al., 2002). Arabidopsis HD2-related proteins (named HDT1–HDT4) are nucleolus localized. Analysis of the Arabidopsis family members identifies HDT1 as a gene important for silencing of one parental set of ribosomal RNA genes in a genetic hybrid, an epigenetic phenomenon called nucleolar dominance (Lawrence et al., 2004). In addition, it has recently been shown that Arabidopsis HDA6, a member of the RPD3-type of HDACs, is also involved in the rDNA silencing (Earley et al., 2006).
Both of the identified plant SIR2-related genes were significantly divergent from yeast SIR2, as they were found in different subclasses. Deletion of yeast SIR2 caused increases of histone acetylation within the rDNA region. However, we did not detect any changes of histone acetylation on rice rDNA in the OsSRT1 RNAi plants, in which overall rDNA expression seemed not to be affected (data not shown). Accordingly, the OsSRT1 protein seemed not to be confined or enriched in the nucleolus (Fig. 2B). Instead, our data showed clear increases of H3K9 acetylation on the tested DNA transposable elements and retroelements in the OsSRT1 RNAi plants. The increases of H3K9 acetylation were in agreement with the transcriptional activation of the DNA and retroelements. In addition, our data showed that the down-regulation of OsSRT1 also affected H3K9 acetylation and expression of hypersensitive response and PCD marker genes, and induced apoptotic cell death on leaves. In agreement with the data, overexpression of OsSRT1 decreased H3K9 acetylation and exhibited enhanced tolerance to an oxidative agent. OsSRT1 is found in the same class (IV) as human nucleus-localized HsSIRT6 and HsSIRT7 proteins, but OsSRT1 is more closely related to HsSIRT6 than HsSIRT7. HsSIRT6 has a weak in vitro HDAC activity. SIRT6 knockout mice display a deficiency in DNA repair and genomic instability (Mostoslavsky et al., 2006). In contrast, no NAD+-dependent HDAC activity is found in SIRT7 that is localized in the nucleolus. SIRT7 has recently been shown to promote rDNA transcription by interacting with RNA polymerase I (Ford et al., 2006). Therefore, it is likely that OsSRT1 has a divergent function from SIR2-related proteins in yeast and mammalian cells.
We showed that OsSRT1 was widely expressed in rice, with highest levels in active cell dividing organs/tissues. Down-regulation of OsSRT1 by RNAi induced lesion mimic cell death and precocious senescence, whereas overexpression showed tolerance to oxidative stress. These data suggest that OsSRT1 is involved in the safeguard against genome instability and/or oxidative stress, required for plant cell growth. Histochemical staining, TUNEL assays, and molecular marker gene analysis demonstrated that cell death was induced in OsSRT1 RNAi plants. The TUNEL positive signals detected in nuclei of the RNAi leaf cells were indicative of DNA fragmentation, supporting the occurrence of apoptotic PCD in the RNAi plants. However, the production of H2O2 and activation of HSR201 suggested that the cell death in the OsSRT1 RNAi plants also resembled hypersensitive response-mediated PCD. Either both types of PCD were induced by OsSRT1 down-regulation, or different triggers of PCD may be interdependent in plants and the downstream effectors of PCD may be shared among different pathways. In plants, it is not known whether PCD occurs in response to DNA damage as a defense mechanism, as DNA damage-induced PCD in mammalian cells requires the activation of p53 (Chowdhury et al., 2006) that has not been identified in plants. Therefore, it is not clear at this stage whether the DNA damage was the cause or the consequence of the PCD induced by the OsSRT1 RNAi. It is also possible that DNA fragmentation was due to genome instability induced by oxidative stress as a result of H2O2 production or transcriptional activation of transposons due to increased H3K9 acetylation, a hallmark of gene activation, as transposons can be activated in response to stress challenges.
Molecular analysis showed an increase of H3K9 acetylation and a decrease of H3K9 dimethylation in OsSRT1 RNAi leaves. Since H3K9 dimethylation is found to be mainly associated with inactive chromatin, these data suggest that OsSRT1 is needed for deacetylation and subsequent dimethylation of H3K9 to inactivate chromosomal domains. The early activation of transposable elements and two of some PCD marker genes, along with the increase of H3K9 acetylation on these genes, suggests that both transposable elements and protein-coding genes may be among the primary targets of OsSRT1. Whether OsSRT1 is directly involved in the repression of the targets requires further analysis.
The activated transposable elements were silent in wild-type plants as judged by the hybridization signals that were at near background levels, suggesting these transposable elements might be within silent heterochromatin domains that are known to be associated with DNA methylation (Tariq et al., 2003). Analysis of genomic DNA isolated from wild-type and OsSRT1 RNAi and overexpression plants revealed two of the tested elements had decreased symmetric cytosine methylation (as revealed by MspI and HpaII digestion) induced by OsSRT1 RNAi and two others had no changes (Supplemental Fig. S2). However, digestion with HaeIII, which could detect asymmetric cytosine methylation, did not reveal clear differences between genome DNAs isolated from the wild-type and the transgenic plants. Therefore, down-regulation of OsSRT1 was likely to have a stochastic effect on DNA methylation. This may lead to suggest that histone deacetylation may play a primary role in OsSRT1-mediated transposon repression. This is in agreement with the observations of Lippman et al. (2003), showing that there are two distinct mechanisms to silence transposons in Arabidopsis, each of which involves different components of chromatin modification and remodeling with histone deacetylation as a common intermediate, as loss of function mutation of HDA6 derepresses most of the tested transposable elements. Our data with OsSRT1 suggest that members from different classes of plant HDAC genes are involved in histone deacetylation required for transposon silencing.
MATERIALS AND METHODS
Gene Cloning and Sequence Analysis
The cDNA fragments of OsSRT1 were amplified from rice (Oryza sativa L. sp. indica) ‘Minghui63’ by RT-PCR. Two micrograms of total RNA from young panicles were reverse transcribed in a total volume of 20 μL with 0.5 μg oligo(dT)15, 0.75 mm dNTPs, 10 mm dithiothreitol, and 100 units SuperScript II RNase H− reverse transcriptase (Invitrogen). The following PCR primers were designed: SRT1-F (5′-GGGGGTACCGAGAGATGTCACTTGGCTATGC-3′; a KpnI site was introduced and is underlined) and SRT1-R (5′-GGGGGATCCCCAGCTTTCACATGCACTAG-3′; a BamHI site was introduced and is underlined). ExTaq DNA polymerase (TaKaRa) was used to amplify with the following cycling profile: 94°C for 3 min; 30 cycles of 94°C for 1 min, 57°C for 1 min, and 72°C for 2 min; and extension at 72°C for 10 min. The PCR product was cloned into the pGEM-T vector to obtain the pT237 clone (Promega) and confirmed by sequencing from both ends.
For sequence analysis, all of the SRT family sequences that were used for sequence alignment and phylogenetic analysis were downloaded from the plant ChromDB database (http://www.chromdb.org/). The sequences of the Sir2 domain (Pfam accession no. PF02146) were searched in the Pfam database (http://pfam.janelia.org/). Phylogeny reconstruction of all Sir2 domain sequence alignments was performed by MEGA 3.1 (Kumar et al., 2004) using the neighbor-joining method with a Poisson correction model and a bootstrap of 500 replicates.
Nuclear Localization
The nuclear localization vector was constructed by replacing the GUS fragment of pCAMBIA1391Xb (CAMBIA) with a ubiquitin promoter-GFP cassette. The coding region of OsSRT1 cDNA was amplified using the following primer pair: FU-F 5′-GGGGAATTCTCGGGAGAAGCTTACTTGATTGAG-3′ (an EcoRI site was introduced and is underlined) and FU-R 5′-GGGGGATCCACTGATCGAAGAAATGGCAAAGG-3′ a BamHI site was introduced and is underlined). The amplified fragment was inserted upstream to and in frame with GFP. The procedure of bombarding onion (Allium cepa) epidermal cells was as described (Dai et al., 2007). The expression of the fusion protein of OsSRT1 and GFP in the onion epidermal cells was observed by a confocal microscope (Leica) 36 h after bombardment.
Vector Construction and Rice Transformation
A 412-bp cDNA fragment of OsSRT1 was amplified using primers RNAi-F (5′-GGGACTAGTGGTACCAGTCCTGCAAGAGTTGCAAC-3′ with a SpeI site, bold letters, and a KpnI site, underlined integrated) and RNAi-R (5′-GGGGAGCTCGGATCCCCAGCTTTCACATGCACTAG-3′ with a SacI site and a BamHI site). PCR products were digested with KpnI/BamHI and SacI/SpeI, respectively, and inserted into pDS1301 (Chu et al., 2006).
The overexpression vector was constructed by directionally inserting the full cDNA sequence (digested with BamHI/KpnI) into the binary vector pU1301, which was modified based on pCAMIA1301 (CAMBIA) and contained a maize (Zea mays) ubiquitin promoter. Agrobacterium tumefaciens (strain EHA105)-mediated transformation of rice plants was conducted according to a published protocol (Lin and Zhang, 2005).
Expression Analysis by Northern Blots, Microarray, and RT-PCR
For northern-blotting analysis, 15 μg of total RNA samples extracted from tissues or organs harvested from field-grown rice plants was separated in 1.2% (w/v) formamide-denaturing agarose gels, before being transferred to nylon membranes. Gene-specific probes were labeled with 32P-dCTP using the Random Primer kit (Invitrogen) and hybridized to the RNA blots. The probe of OsSRT1 was digested from pT237 plasmid with KpnI and SpeI, a fragment of 635 bp of the cDNA.
For microarray analysis, transgenic and wild-type seedlings were grown in half-strength Murashige and Skoog medium under a 16-h-light/8-h-dark cycle at 25°C for 11 d, for three biological repeats. RNA samples were extracted using TRIzol (Invitrogen) as described by the manufacturer. Hybridization with Affymetrix GeneChip Rice Genome Arrays was performed at CapitalBio Corporation. The dataset was normalized with the option of all probe sets scaled to the target signal of 100. The genes with expression calls as absent from at least 11 arrays were filtered, resulting in 17,806 genes for further analyses. The significance analysis of microarrays (SAM) Excel add-in (Tusher et al., 2001) was used to identify significantly differentially expressed genes between the control and RNAi seedlings. The imputation engine was set as 10-nearest neighbor imputer and the number of permutations was 100. The delta value in the SAM was adjusted so that the estimated false discovery rate was <5% for significant genes.
For semiquantitative RT-PCR analysis, 2 μg of total RNA was reverse-transcribed in a total volume of 20 μL with 0.5 μg oligo(dT)15, 0.75 mm dNTPs, 10 mm dithiothreitol, and 100 units of SuperScript II RNase H− reverse transcriptase (Invitrogen). PCR was performed in a total volume of 20 μL with 1 μL of the RT reactions, 0.2 μm gene-specific primers, and 1 unit of rTaq (TaKaRa). Twenty-five to 30 cycles were performed. Rice actin cDNA was used as internal control. The sequences of the used primers are listed Supplemental Table S1.
DAB Assays
The DAB uptake method (Thordal-Christensen et al., 1997) was used to detect H2O2. Ten-day-old seedlings were placed in DAB dissolved in 10 mm ascorbic acid (1 mg/mL) for 2 h. The seedlings were then boiled in 96% ethanol for 10 min and stored in 96% ethanol. H2O2 production was visualized as reddish-brown coloration.
TUNEL Assays
The TUNEL assays were performed using the In Situ Cell Death Detection Kit-Fluorescein (Roche Diagnostics). Leaf tissues from wild-type and RNAi plants were fixed in 4% paraformaldehyde in 0.1 m phosphate-buffered saline (pH 7.2) containing 0.1% (v/v) Triton X-100 and Tween 20 at 4°C overnight and embedded in paraplasts. Eight-micrometer sections on glass slides were dewaxed in xylene, rehydrated, and then pretreated with 20 mg/mL proteinase K in 10 mm Tris-Cl, pH 7.5, for 20 min at room temperature. Two slides, treated with 1,500 units/mL DNase I in 50 mm Tris-Cl, pH 7.5, 1 mm MgSO4, and 1 mg/mL bovine serum albumin for 20 min at room temperature, served as positive controls. Two slides, labeled in the absence of the terminal deoxyribonucleotidyl transferase enzyme, served as negative controls. Vectashield mounting medium (Vector Laboratories) and protease inhibitors (1 mg/mL; Sigma) were used to mount the slides before they were viewed and photographed with a Leica microscope.
Paraquat Treatment
For paraquat treatments, leaves of 14-d-old plants (three replications, 50 per treatment) were exposed to a surface application of 10 μm paraquat (Sigma) in a 0.1% solution of the nonionic surfactant Ortho X77 (Valent USA) 5 h during the light period. The controls were treated with 0.1% surfactant alone (Donahue et al., 1997).
Western-Blot Analysis
Rice leaf histone protein extraction was performed as described (Tariq et al., 2003). After washing in acetone and dried, the proteins were resuspended in Laemmli sample buffer (62.5 mm Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, and 10% β-mercaptoethanol), then separated on a 16% SDS-PAGE and transferred to an Immobilon-P PVDF transfer membrane (Millipore). The membrane was blocked with 2% bovine serum albumin in phosphate-buffered saline (pH 7.5), and incubated overnight with primary antibodies, such as anti-acetylated histone H3, anti-dimethyl-histone H3K9, and anti-acetyl-histone H3K9 (from Upstates; catalog nos. 06–599, 07–441, and 07–352, respectively) in a 1:5,000 dilution at room temperature. After three washes (30 min each), the secondary antibody (goat anti-rabbit IgG [SouthernBiotech]) at 1:10,000 dilution was used. Visualization was performed using the SuperSignal West Pico kit (Pierce) according to the manufacturer's instructions.
ChIP Assay
ChIP assays were performed as described (Benhamed et al., 2006). Immunoprecipitated DNA was analyzed by real-time PCR (Applied Biosystems 7500). Primers were designed by PRIMER EXPRESS 2.0 software (PE Applied Biosystems) to amplify 80- to 120-bp products. Products were measured by SYBR green fluorescence (Applied Biosystems) in 25-μL reactions; all primers were annealed at 58°C. Data analyses with 2−ΔΔCt method were performed as described (Livak and Schmittgen, 2001). Real-time PCR primers are listed in Supplemental Table S2.
DNA Methylation Analysis
Genomic DNA (1 μg) was digested for 6 h at 37°C with 30 units of HpaII, MspI, or HaeIII. Five percent of the digested and input (undigested) DNA was analyzed by PCR (Onodera et al., 2005). PCR conditions were as follows: 5 min at 96°C, followed by 35 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min. Primers used for PCR are listed in Supplemental Table S3.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number XP_471492.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Transgenic siblings without OsSRT1 overexpression showed no enhanced tolerance to paraquat.
Supplemental Figure S2. Analysis of cytosine methylation of transposons in OsSRT1 overexpression or RNAi plants.
Supplemental Table S1. Primers used for RT-PCR analysis.
Supplemental Table S2. Primers used for real-time PCR analysis.
Supplemental Table S3. Primers used for DNA methylation analysis.
Supplementary Material
Acknowledgments
We thank Li Xianhua for technical assistance, Xu Caiguo for rice field management, and Song Huazhi for assistance with confocal microscopy. We acknowledge Qiu Deyun and Xie Weibo for help in microarray data analysis.
This work was supported by grants from the National Special Key Program of Rice Functional Genomics and the National Natural Science Foundation of China.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Dao-Xiu Zhou (dao-xiu.zhou@u-psud.fr).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Benhamed M, Bertrand C, Servet C, Zhou DX (2006) Functional interaction of histone acetylation enzymes in light-regulation of gene expression in Arabidopsis. Plant Cell 18 2893–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander G, Guarente L (2004) The Sir2 family of protein deacetylases. Annu Rev Biochem 73 417–435 [DOI] [PubMed] [Google Scholar]
- Brodersen P, Petersen M, Pike HM, Olszak B, Skov S, Odum N, Jorgensen LB, Brown RE, Mundy J (2002) Knockout of Arabidopsis accelerated-cell-death11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes Dev 16 490–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozza MJ, Utley RT, Workman JL, Côté J (2003) The diverse function of histone acetyltransferase complexes. Trends Genet 19 321–329 [DOI] [PubMed] [Google Scholar]
- Chowdhury I, Tharakan B, Bhat GK (2006) Current concepts in apoptosis: the physiological suicide program revisited. Cell Mol Biol Lett 11 506–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Yuan M, Yao J, Ge X, Yuan B, Xu C, Li X, Fu B, Li Z, Bennetzen JL, et al (2006) Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev 20 1250–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernic P, Huang HC, Marco Y (1996) Characterization of hsr201 and hsr515, two tobacco genes preferentially expressed during the hypersensitive reaction provoked by phytopathogenic bacteria. Plant Mol Biol 31 255–265 [DOI] [PubMed] [Google Scholar]
- Dai M, Hu Y, Zhao Y, Liu H, Zhou D-X (2007) A WUSCHEL-LIKE HOMEOBOX gene represses a YABBY gene expression required for rice leaf development. Plant Physiol 144 380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JL, Okpodu CM, Cramer CL, Grabau EA, Alscher RG (1997) Responses of antioxidants to paraquat in pea leaves (relationships to resistance). Plant Physiol 113 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley K, Lawrence RJ, Pontes O, Ruether R, Enciso AJ, Silva M, Neves N, Gross M, Viegas W, Pikaard CS (2006) Erasure of histone acetylation by Arabidopsis HDA6 mediates large-scale gene silencing in nucleolar dominance. Genes Dev 20 1283–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L (2006) Mammalian Sir2 homolog SRT7 is an activator of RNA polymerase I transcription. Genes Dev 20 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA (2000) Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273 793–798 [DOI] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP (2006) Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev 20 2913–2921 [DOI] [PubMed] [Google Scholar]
- Kandel S, Morant M, Benvenist I, Blée E, Werck-Reichhart D, Pinot F (2005) Cloning, functional expression, and characterization of CYP709C1, the first sub-terminal hydroxylase of long chain fatty acid in plants. J Biol Chem 280 35881–35889 [DOI] [PubMed] [Google Scholar]
- Kong L, Anderson JM, Ohm HW (2005) Induction of wheat defense and stress-related genes in response to Fusarium graminearum. Genome 48 29–40 [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 5 150–163 [DOI] [PubMed] [Google Scholar]
- Lawrence RJ, Earley K, Pontes O, Silva M, Chen JZ, Neves N, Viegas W, Pikaard CS (2004) A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol Cell 13 599–609 [DOI] [PubMed] [Google Scholar]
- Lin YJ, Zhang Q (2005) Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep 23 540–547 [DOI] [PubMed] [Google Scholar]
- Lippman Z, May B, Yordan C, Singer T, Martienssen R (2003) Distinct mechanisms determine transposon inheritance and methylation via small interfering RNA and histone modification. PLoS Biol 1 420–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Lusser A, Brosch G, Loidl A, Haas H, Loidl P (1997) Identification of maize histone deacetylase HD2 as an acidic nucleolar phosphoprotein. Science 277 88–91 [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, et al (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124 315–329 [DOI] [PubMed] [Google Scholar]
- Onodera Y, Haag JR, Ream T, Nunes PC, Pontes O, Pikaard CS (2005) Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 120 613–622 [DOI] [PubMed] [Google Scholar]
- Pandey R, Muller A, Napoli CA, Selinger DA, Pikaard CS, Richards EJ, Bender J, Mount DW, Jorgensen RA (2002) Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res 30 5036–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier D, Gan S, Amasino RM, Roby D, Lam E (1999) Markers for hypersensitive response and senescence show distinct patterns of expression. Plant Mol Biol 39 1243–1255 [DOI] [PubMed] [Google Scholar]
- Robyr D, Suka Y, Xenarios I, Kurdistani SK, Wang A, Suka N, Grunstein M (2002) Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 109 437–446 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403 41–45 [DOI] [PubMed] [Google Scholar]
- Tariq M, Saze H, Probst AV, Lichota J, Habu Y, Paszkowski J (2003) Erasure of CpG methylation in Arabidopsis alters patterns of histone H3 methylation in heterochromatin. Proc Natl Acad Sci USA 100 8823–8827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. Plant J 11 1187–1194 [Google Scholar]
- Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.