Abstract
The pbs3-1 mutant, identified in a screen for Arabidopsis (Arabidopsis thaliana) mutants exhibiting enhanced susceptibility to the avirulent Pseudomonas syringae pathogen DC3000 (avrPphB), also exhibits enhanced susceptibility to virulent P. syringae strains, suggesting it may impact basal disease resistance. Because induced salicylic acid (SA) is a critical mediator of basal resistance responses, free and glucose-conjugated SA levels were measured and expression of the SA-dependent pathogenesis-related (PR) marker, PR1, was assessed. Surprisingly, whereas accumulation of the SA glucoside and expression of PR1 were dramatically reduced in the pbs3-1 mutant in response to P. syringae (avrRpt2) infection, free SA was elevated. However, in response to exogenous SA, the conversion of free SA to SA glucoside and the induced expression of PR1 were similar in pbs3-1 and wild-type plants. Through positional cloning, complementation, and sequencing, we determined that the pbs3-1 mutant contains two point mutations in the C-terminal region of the protein encoded by At5g13320, resulting in nonconserved amino acid changes in highly conserved residues. Additional analyses with Arabidopsis containing T-DNA insertion (pbs3-2) and transposon insertion (pbs3-3) mutations in At5g13320 confirmed our findings with pbs3-1. PBS3 (also referred to as GH3.12) is a member of the GH3 family of acyl-adenylate/thioester-forming enzymes. Characterized GH3 family members, such as JAR1, act as phytohormone-amino acid synthetases. Thus, our results suggest that amino acid conjugation plays a critical role in SA metabolism and induced defense responses, with PBS3 acting upstream of SA, directly on SA, or on a competitive inhibitor of SA.
Disease resistance in plants is often dependent on recognition of infecting pathogens by specific disease resistance (R) proteins (Jones and Dangl, 2006). Once activated, R proteins trigger a complex cascade of defense responses, including production of activated oxygen species, fortification of cell walls, accumulation of antimicrobial proteins, known as pathogenesis-related (PR) proteins, production of antimicrobial secondary metabolites, and localized programmed cell death, known as the hypersensitive response (HR; Hammond-Kosack and Jones, 1996). The specific signal transduction steps leading to these various responses are poorly understood, although forward-genetic screens have identified many potential regulators of these responses (Innes, 1998; Glazebrook, 2005).
A key second messenger involved in inducing production of PR proteins and amplifying the oxidative burst is salicylic acid (SA; Ryals et al., 1996; Shirasu et al., 1997). SA levels in uninfected dicot plants are normally very low, but rapidly increase upon infection with avirulent pathogens (i.e. those that activate an R protein; Klessig and Malamy, 1994). Virulent pathogens also induce accumulation of SA, but not as rapidly as avirulent strains (Zhou et al., 1998; Shapiro and Gutsche, 2003; S.K. Marr and M.C. Wildermuth, unpublished data). In Arabidopsis (Arabidopsis thaliana), the bulk of pathogen-induced SA accumulates as the SA-O-β-glucoside (SAG). Mutations that reduce SA production (Nawrath and Metraux, 1999; Dewdney et al., 2000) or impair SA perception (Cao et al., 1994; Delaney et al., 1995; Shah et al., 1997) increase susceptibility to both virulent and avirulent pathogens, indicating that SA contributes to both basal and R-protein-mediated resistance.
To identify additional components of the R-protein signal transduction pathway, Warren et al. (1999) screened for Arabidopsis mutants that displayed enhanced susceptibility after inoculation with an avirulent strain of Pseudomonas syringae pv tomato (Pst DC3000 [avrPphB]). Resistance to this strain by Arabidopsis var. Columbia-0 (Col-0) is mediated by the RPS5 gene (Simonich and Innes, 1995), which encodes a member of the nucleotide-binding site Leu-rich repeat family of R proteins (Warren et al., 1998). This mutant screen led to the identification of several susceptible alleles of RPS5 plus mutations in three additional genes, which were named PBS1, PBS2, and PBS3 for avrPphB susceptible (Warren et al., 1999). The isolation and characterization of PBS1 and PBS2 have been reported previously (Swiderski and Innes, 2001; Tornero et al., 2002). Here, we describe the isolation and characterization of PBS3.
Warren et al. (1999) reported that the pbs3 mutant displayed enhanced disease susceptibility to both virulent and avirulent Pst DC3000 strains, including DC3000 (avrPphB) and DC3000 (avrRpt2; Warren et al., 1999). This phenotype is similar to that reported for SA-deficient Arabidopsis mutants, such as enhanced disease susceptibility5 (eds5)/SA induction deficient1 (sid1; Rogers and Ausubel, 1997; Nawrath and Metraux, 1999; Nawrath et al., 2002) and sid2/eds16 (Nawrath and Metraux, 1999; Dewdney et al., 2000; Wildermuth et al., 2001). Here, we report that pbs3-1 contains two point mutations in the C-terminal region of the protein encoded by At5g13320. Additional analyses with T-DNA insertion (pbs3-2) and transposon insertion (pbs3-3) mutants in At5g13320 were used to further confirm the findings with pbs3-1. PBS3 (also referred to as GH3.12) is a member of the GH3 protein family of acyl-adenylate/thioester-forming enzymes (Staswick et al., 2002). Characterized GH3 family members, such as JAR1, act as phytohormone-amino acid synthetases (Staswick et al., 2002, 2005; Staswick and Tiryaki, 2004). We found that pbs3 mutants are compromised in both pathogen-induced accumulation of the SAG and expression of the SA-dependent marker gene PR1 (At2g14610). Surprisingly, the level of free SA was about 2-fold higher than wild-type plants. Exogenous application of SA was sufficient to restore SAG accumulation, PR1 expression, and enhanced resistance to virulent DC3000. Because mutations in PBS3 impact SAG accumulation, SA-dependent gene expression, and disease resistance, PBS3 plays an important role in SA metabolism. Herein, we present the above findings and discuss possible biochemical functions for PBS3 consistent with its observed functional impact and its putative biochemical activity as a GH3 family member.
RESULTS
Positional Cloning of PBS3
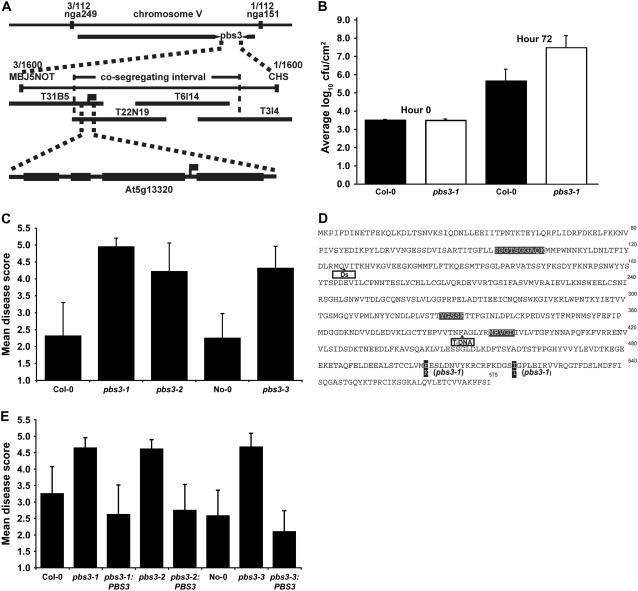
Warren et al. (1999) mapped the pbs3 mutation to chromosome 5 in the vicinity of microsatellite marker nga249 at 28.4 cM on the genetic map. To refine this position, an F2 mapping population was inoculated with Pst DC3000 (avrRpt2), and plants displaying disease symptoms were identified and then scored for PCR-based markers adjacent to nga249. These analyses placed the pbs3 mutation between microsatellite markers nga249 and nga151, a map distance of approximately 5.9 cM (Fig. 1). We then performed high-resolution genetic mapping by using PCR to preselect F2 plants containing recombination events between nga249 and nga151. From approximately 800 plants, we identified 70 informative recombinants. These 70 lines were scored for resistance to Pst DC3000 (avrRpt2) and for additional markers located between nga249 and nga151. Scores were confirmed in F3 families derived from each F2 plant. Analysis of these lines placed the pbs3 mutation between markers MBJ5NOT and CHS1 (Fig. 1A).
Figure 1.
Identification of the PBS3 gene. A, Genetic and physical map of the region containing PBS3. B, Growth of P. syringae strain ES4326 in wild-type and pbs3-1 mutant leaves. C, Semiquantitative disease scores of wild-type and pbs3 mutant plants inoculated with ES4326. D, Sequence of PBS3 highlighting locations of pbs3-1 mutations, Ds, and T-DNA insertions, along with locations of conserved domains and ATP-binding site. E, Complementation of pbs3 mutants with the wild-type PBS3 gene (semiquantitative disease scores).
The distance between MBJ5NOT and CHS1 is approximately 300 kb. This interval contains 80 predicted genes. We obtained T-DNA insertion lines that disrupted 40 of these genes from the Arabidopsis Biological Resource Center (ABRC; Alonso et al., 2003) and assayed these lines for resistance to Pst DC3000 (avrRpt2) using dip inoculations. Among the T-DNA insertion lines tested, SALK line 018225 in predicted gene At5g13320 displayed small water-soaked lesions in the inner rosette leaves (data not shown) similar to those observed on pbs3-1 mutant plants. However, we found these symptoms to be variable among individual plants and from 1 d to another.
Because of this variation, we tested several alternative inoculation methods and pathogen strains to identify a more reproducible assay. We found that the standard eds assay developed by Glazebrook et al. (1996) gave us the most robust results. This assay employs a needleless syringe to inoculate individual leaves with a low titer of P. syringae pv maculicola strain ES4326 (Psm). Using this assay, wild-type Col-0 plants produce little to no disease symptoms, whereas pbs3-1 mutants produce marked yellowing with occasional lesions (data not shown). We developed a semiquantitative disease phenotype scale to score these symptoms, ranging from a value of 1 (no symptoms) to 5 (complete collapse of the inoculated region; Supplemental Fig. S1A). We validated this scoring system by also titering bacterial populations in infected leaves of both wild-type and pbs3-1 mutant plants at 3 d postinoculation (dpi). Figure 1B shows that the pbs3-1 mutant supported significantly more growth of strain ES4326 than wild-type Col-0.
Using this scoring system, we found that SALK T-DNA insertion line 018225 was significantly more susceptible than wild-type plants and similar to the pbs3-1 mutant (Fig. 1C). We also obtained a Dissociation (Ds) transposon line from the RIKEN collection with an insertion in At5g13320 (Kuromori et al., 2004). This line, which is in the Nössen (No-0) genetic background, was also significantly more susceptible to ES4326 than its wild-type sibling (Fig. 1C). We therefore named these insertion alleles pbs3-2 and pbs3-3, respectively.
The above data strongly suggested that At5g13320 corresponded to PBS3. To confirm this, we amplified At5g13320 from the pbs3-1 mutant and sequenced it. We found two point mutations that substitute a Lys for a Glu (E502K) and a Thr for an Ile (I519T; Fig. 1D), indicating that At5g13320 indeed corresponds to PBS3.
Further support for this conclusion was obtained by complementing the pbs3-1 and insertion mutants by transformation with a wild-type genomic copy of PBS3, extending from 1,020 bp upstream of the translation initiation site to 127 bp downstream of the stop codon. Disease symptoms of T1 transformant plants were quantified and compared with that of wild-type and mutant plants. As expected, the transformants showed significant reduction in disease scores, confirming that At5g13320 is PBS3 (Fig. 1E).
We then examined PBS3 transcripts in the pbs3-1 and pbs3-2 mutants. As shown in Supplemental Figure S2, PBS3 transcript was detected in wild-type and pbs3-1 plants in response to Pst DC3000 (avrRpt2), but not in the insertion mutant pbs3-2 using primers that flank intron 3, the location of the T-DNA insertion (see Fig. 1D).
PBS3 Belongs to the GH3 Family of Acyl Adenylases
At5g13320 is a member of the GH3 multigene family that consists of 19 family members in Arabidopsis var. Col-0 (Staswick et al., 2002). The first GH3 gene described was isolated from soybean (Glycine max) as an early auxin-responsive gene (Hagen and Guilfoyle, 1985). Since then, homologs have been identified in many plants, including Arabidopsis, rice (Oryza sativa), Gossypium spp., Lycopersicon spp., Populus spp., Pinus spp., and the moss Physcomitrella patens based on ESTs or sequence data (Terol et al., 2006) and in some bacteria (R. Okrent and M. Wildermuth, unpublished data). The GH3 proteins are members of a large enzyme superfamily of acyl-adenylate/thioester-forming enzymes that catalyze a variety of reactions with a common first step: transfer of AMP from ATP to the carboxylic acid group of an acyl substrate forming an activated acyl-adenylate intermediate (Staswick et al., 2002). Characterized family members have been shown to catalyze the ATP-dependent conjugation of amino acids to the phytohormones jasmonic acid (JA) or indole acetic acid (IAA) with mutants exhibiting altered phytohormone phenotypes (Staswick et al., 2002, 2005; Staswick and Tiryaki, 2004). For example, the jar1 mutant was isolated as a jasmonate-insensitive mutant and displays enhanced susceptibility to necrotrophic, but not biotrophic, pathogens (Staswick et al., 1998).
Phylogenetic analysis of the 19 AtGH3 family members identified three sequence homology groups, with the known substrate specificity corresponding to their phylogenetic relationships: group I members, which include JAR1, a JA-amino acid synthetase; group II members, which are capable of adenylating IAA; and group III members, which include PBS3 (GH3.12) and act on unknown substrates (Staswick et al., 2002, 2005; Staswick and Tiryaki, 2004). Recombinant PBS3 did not show significant activity with any of the tested substrates—JA, JA methyl ester, IAA, abscisic acid, 1-aminocyclopropane-1-carboxylic acid, gibberellic acid, 2-oxophytodienoic acid, linolenic acid, or SA (Staswick et al., 2002)—suggesting that the recombinant protein used in these assays was inactive or that the correct substrate was not provided.
As shown in Figure 1D and Supplemental Figure S3, PBS3 has all three AMP-binding motifs that are necessary for adenylation. Residues of import for substrate specificity have not yet been determined, but are likely to reside near the AMP-binding motifs as has been shown for other members of the acyl-adenylate/thioester-forming superfamily (Gulick et al., 2004; Nakatsu et al., 2006). The pbs3-1 point mutations result in two amino acid changes (E502K and I519T) that are located in the C-terminal domain of the PBS3 protein, not in or near the AMP-binding motifs. Both residues are highly conserved in Arabidopsis GH3 family members, with amino acids containing negatively charged R groups (E or D) at residue 502 and the nonpolar aliphatic R-group members I or V at residue 519. The C-terminal region of these GH3 proteins contains no known protein domains or motifs. It is possible that this region facilitates conjugation of the amino acid to the activated AMP intermediate, although this remains to be determined.
The pbs3-1 Mutation Impairs Accumulation of SAG and PR1 by Pathogens
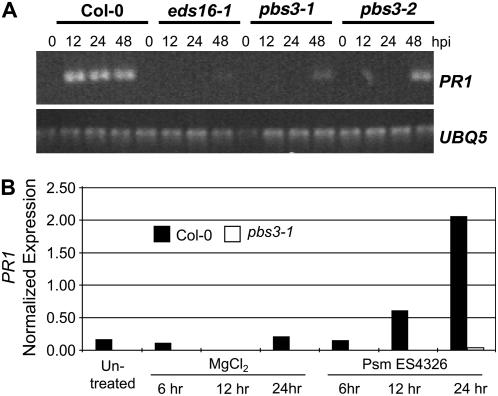
The enhanced susceptibility of pbs3 mutant plants to both virulent and avirulent pathogens is similar to previously described eds mutants, many of which are compromised in the accumulation of SA and the expression of the SA-dependent marker PR1 (Glazebrook et al., 1996; Rogers and Ausubel, 1997; Dewdney et al., 2000). We therefore assayed levels of PR1 transcript in the pbs3 mutant before and after infection with two different P. syringae strains. As seen in Figure 2A, the avirulent Pst strain DC3000 (avrRpt2) strongly induced PR1 by 12 h postinoculation (hpi) in wild-type plants, whereas PR1 transcript was not detected in pbs3-1 or pbs3-2 plants until 48 hpi. Similarly, the virulent strain ES4326 induced PR1 highly by 24 hpi in wild-type plants, whereas transcript levels were at least 50-fold lower in the pbs3-1 mutant (Fig. 2B).
Figure 2.
Pathogen-induced expression of PR1 is delayed and reduced in the pbs3 mutant. A, RT-PCR analysis of PR1 expression in wild type, pbs3 mutants, and eds16-1 in response to Pst DC3000 (avrRpt2; OD600 0.0001). B, qRT-PCR analysis of PR1 expression in wild type and the pbs3-1 mutant in response to Psm ES4326 (OD600 0.002).
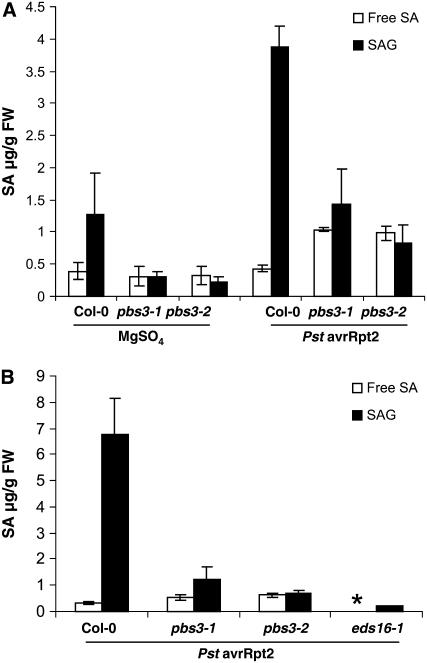
The reduced levels of PR1 transcript in pbs3 mutants suggested that production and/or perception of SA might be compromised. We therefore measured both free SA and SAG levels in pbs3-1 and pbs3-2 mutant plants before and 24 hpi with Pst DC3000 (avrRpt2). At this time point, wild-type plants exhibit significant expression of PR1, whereas pbs3 mutants do not (Fig. 2A). We found significant accumulation of SAG in wild-type plants, with an average 5-fold reduction in accumulated SAG in pbs3 mutant plants (Fig. 3A; Supplemental Table S1). Surprisingly, the pbs3 mutants accumulated approximately 2-fold more free SA than wild type at this time point (Fig. 3A; Supplemental Table S1). Because the majority of SA is found as SAG, the total SA present in the pbs3 mutants 24 hpi was significantly lower (2- to 3-fold) than in wild-type plants. Mutants with reduced (e.g. pad4 [Zhou et al., 1998]) or abrogated (e.g. ics1/sid2-1/eds16-1 [Nawrath and Metraux, 1999; Dewdney et al., 2000; Wildermuth et al., 2001]) induction of SA and SAG in response to pathogens have been described. In contrast, here, we report on a mutant exhibiting elevated free SA with dramatically reduced SAG accumulation in response to pathogens. Direct comparison of PR1 induction and SA and SAG accumulation in the pbs3 mutants with the SA biosynthetic mutant eds16-1 confirmed that, whereas pathogen-induced PR1 and SAG accumulation are abrogated in the eds16-1 mutant, they are dramatically reduced in the pbs3 mutants (Figs. 2A and 3B).
Figure 3.
SAG accumulation is dramatically reduced in pbs3 mutant plants in response to Pst DC3000 (avrRpt2). A, Free SA and SAG levels in wild-type Col-0, pbs3-1, and pbs3-2 inoculated with Pst DC3000 (avrRpt2; OD600 0.0001), or with 10 mm MgSO4 at 24 hpi. B, Free SA and SAG levels in wild type, pbs3-1, and pbs3-2 compared with the SA biosynthetic mutant eds16-1 at 24 hpi with Pst DC3000 (avrRpt2; OD600 0.0001). Each bar represents the average of three replicates ± sd. *, Below detection limit.
These results suggest that the PBS3 protein contributes to, or regulates, SAG biosynthesis and total SA accumulation, as well as expression of the SA-dependent marker PR1 and resistance to P. syringae pathogens. The finding that free SA levels were elevated (not reduced) suggests that PBS3 might act directly on SA and that the product formed by PBS3 impacts accumulation of the SA Glc conjugate and expression of PR1.
Exogenous SA Restores SAG Accumulation, PR1 Expression, and Resistance
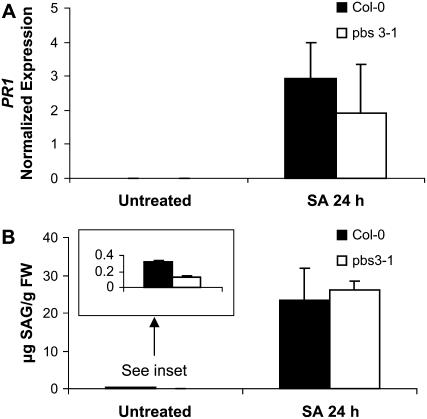
To determine whether pbs3 mutants have a defect in processing free SA and whether this processing is required for wild-type pathogen-induced accumulation of SAG and PR1, we treated wild-type and pbs3-1 mutant plants with exogenous SA. Figure 4A shows that application of 2.5 mm SA to pbs3-1 mutant leaves restores induced PR1 expression at levels similar to wild-type 24 h posttreatment (hpt) with SA. This PR1 induction is dramatic, with relative increases in PR1 expression at 24 hpt (compared to untreated) of >150-fold for all samples of pbs3-1 and wild type.
Figure 4.
Exogenous treatment with 2.5 mm SA of pbs3-1 restores induced PR1 expression and SAG accumulation at 24 hpt. A, qRT-PCR analysis of PR1 expression in pbs3-1 and wild type in response to exogenous SA treatment. Shown is the average with sd of three biological replicates, from independent experiments. B, SAG levels in pbs3-1 and wild-type plants in response to exogenous SA treatment. Each bar represents the average of three replicates with sd.
SAG formation was also comparable for pbs3-1 and wild-type leaves (Fig. 4B) at 24 hpt with SA. SAG is calculated by subtracting measured free SA (no hydrolysis) from measured total SA (after hydrolysis of SAG to SA), as described in “Materials and Methods.” Significant SAG (approximately 25 μg/g fresh weight) was formed in leaves of wild-type and pbs3-1 mutants 24 hpt with exogenous SA, resulting in reproducible differences in measured total − free SA values. It should be noted, however, that direct measurement of SAG would need to be performed to ascertain subtle changes in SAG in response to exogenous SA. To determine whether exogenous SA treatment conferred enhanced disease resistance, we subsequently (24 hpt) inoculated leaves with Psm ES4326 (OD600 = 0.0002) and assessed bacterial growth immediately after inoculation and at 3 dpi. We also performed parallel experiments using the active SA analog 2,6-dichloroisonicotinic acid (INA; 0.65 mm) for the pretreatment instead of SA (4 mm). Bacterial growth at 3 dpi was limited by pretreatment of pbs3-1 and wild-type leaves with the SA analog INA (data not shown). Although SA pretreatment also conferred resistance to Psm ES4326 for both the pbs3-1 and wild-type plants, the results were less consistent than with INA, perhaps due to secondary effects from the high concentration of SA (4 mm) employed in these experiments.
Given these findings, it appears that pbs3 mutants are not defective in the perception or processing of SA and that PBS3 function is not required for the conversion of SA to SAG or for the SA-dependent induction of PR1 expression.
PBS3 Is Induced by Pathogens and Is Highly Correlated with ICS1
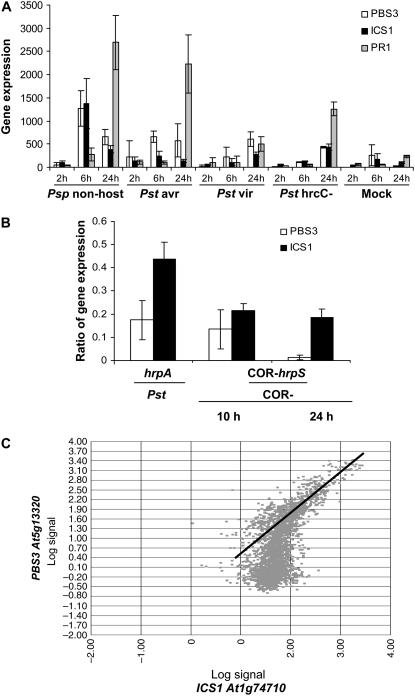
Analysis of publicly available Arabidopsis ATH1 Affymetrix GeneChip microarray data (Craigon et al., 2004; Zimmermann et al., 2004) revealed that PBS3 (At5g13320) expression is typically quite low, but is strongly induced by Pseudomonas pathogens with some induction by oxidative stresses such as ozone and UVB. As shown in Figure 5A, PBS3 is expressed most rapidly and strongly in response to nonhost strains of P. syringae and, to a somewhat lesser degree, by avirulent and virulent strains. Furthermore, the type III secretion system (TTSS) is required for maximal induction of PBS3 expression as Pst hrcC mutants, which cannot deliver type III effectors to host cells, do not induce PBS3 as strongly as does Pst (Fig. 5A). Average ratios for PBS3 gene expression in the Pst hrcC mutant compared with Pst were 0.35, 0.48, and 0.7 at 2, 6, and 24 hpi, respectively. Analysis of the Thilmony et al. (2006) microarray dataset provides even stronger evidence in support of TTSS involvement because PBS3 induction is dramatically reduced in the Pst, hrpS, and hrpA mutants, which cannot express or secrete TTSS effector genes and proteins, respectively (Fig. 5B).
Figure 5.
Comparative expression of PBS3 and ICS1 in response to bacteria. A, Comparative expression of PBS3, ICS1, and PR1 in response to P. syringae. Expression in response to nonhost (Psp), avirulent (Pst DC3000 [avrRpt2]), virulent (Pst DC3000), type III secretion-deficient mutant (Pst DC3000 [hrcC−]), and mock MgCl2 treatment is shown. Expression data were acquired from NASCArrays (Craigon et al., 2004) for the experiment performed by Nürnberger (NASCArrays-120). Dosage for each inoculation was 1 × 108 cfu/mL. B, Ratio of expression induced by type III secretion-deficient versus type III secretion wild-type P. syringae strains for the PBS3 and ICS1 genes. The Pst hrpA and hrpS mutants are deficient in type III secretion, and Pst COR− is deficient in the synthesis of the toxin coronatine. Expression data were acquired from NASCArrays for the experiment performed by Underwood (NASCArrays-340), published in Thilmony et al. (2006). The Pst and Pst hrpA inoculations were performed at a dosage of 1 × 108 bacteria/mL and samples were collected after 7 h. The Pst COR− hrpS and COR− inoculations used 5 × 107 cfu/mL with collection 10 hpi and 1 × 106 cfu/mL with collection 24 hpi, respectively. C, NASCArray gene correlation plot for log PBS3 (At5g13320) versus log ICS1 (At1g74710) expression using the full ATH1 Affymetrix wild-type NASCArray dataset. Perfect positive correlation would be an upward diagonal line. Note the high degree of correlation in slides (GeneChips) with significant induction of both genes.
As shown in Figure 5, expression of the pathogen-induced SA biosynthetic gene ICS1 (SID2/EDS16) parallels that of PBS3. Because PBS3 impacts pathogen-induced free SA and SAG accumulation, as well as expression of the SA-dependent marker PR1, we assessed whether ICS1 and PBS3 expression are correlated across all experiments using wild-type Col-0 plants in the NASCArray Database (821 ATH1 GeneChips; Craigon et al., 2004). We found that PBS3 expression is well correlated with ICS1 (At1g74710), with a Pearson correlation coefficient of 0.63 (see “Materials and Methods”), whereas the Pearson correlation coefficient for PR1 (At2g14610) with ICS1 is 0.42. As shown in Figure 5C, when significant expression of ICS1 and PBS3 is observed, their expression is very highly correlated. Indeed, PBS3 is the second most correlated gene with ICS1 of the >22 K genes on the ATH1 GeneChip, whereas PR1 is the 140th most correlated gene. We observed no significant correlation in expression between PBS3 and any of the other Arabidopsis GH3 family members, suggesting that PBS3 plays a unique role among its family.
DISCUSSION
In Arabidopsis and many other plants, SA is a key molecule that activates plant defense genes and its accumulation is known to be necessary for local and systemic acquired resistance (Durrant and Dong, 2004). Although SA is necessary for triggering plant defense pathways, an excess amount of free SA may be phytotoxic (e.g. Lee et al., 1995; Kenton et al., 2000). Plants regulate free SA levels in part by glucosylation forming SAG as the dominant form of SA detected in plants (Enyedi et al., 1992; Malamy et al., 1992). Infection by pathogens such as Tobacco mosaic virus and P. syringae pathovars rapidly induces the accumulation of free SA and SAG (Malamy et al., 1992; Chong et al., 2001; Shapiro and Gutsche, 2003). The conversion of free SA to SAG has been followed using radiolabeled SA (Dean et al., 2005) and appears to be catalyzed by UDP-Glc:SA glucosyltransferases (SAGT). In tobacco (Nicotiana tabacum) and Arabidopsis, putative SAGTs have been identified based on correlation of expression with accumulation of SAG, induction of SAGT expression by free SA, and the ability of these enzymes to catalyze the formation of SAG in vitro (Enyedi and Raskin, 1993; Lee and Raskin, 1999; Lim et al., 2002; Song, 2006). However, sagt mutants deficient in SAG formation have not yet been reported.
Although SA appears to be synthesized in the chloroplast (Strawn et al., 2007), SAG is formed in the cytoplasm and then transported into the vacuole (Dean and Mills, 2004; Dean et al., 2005). The vacuolar localization of SAG suggests that it is primarily a storage form of SA. Whether SAG can be exported back out of the vacuole is not known. Because SAG can be rapidly hydrolyzed to form free SA by endogenous hydrolases, SAG is hypothesized to function as an inactive pool for the rapid sustained induction of systemic acquired resistance. In support of this function, exogenous application of SAG, but not the nonhydrolyzable SAG analog thio-SAG, results in the accumulation of free SA and expression of PR1 (Hennig et al., 1993).
The pbs3 mutants exhibit approximately 5-fold reduction in pathogen-induced SAG accumulation compared to wild-type Arabidopsis plants (Fig. 3; Supplemental Table S1). In contrast to SAG levels, the level of free SA is significantly higher in the pbs3 mutant relative to wild type at 24 hpi with Pst (avrRpt2). This unusual phenotype suggests that PBS3 may regulate the conversion of SA to SAG. Total SA levels (SA + SAG) are 2- to 3-fold lower in pathogen-infected pbs3 mutant plants than in wild type; thus, PBS3 not only affects accumulation of SAG, but somehow impacts the overall metabolism of SA such that net SA biosynthesis is reduced or SA turnover is increased.
Pathogen-induced expression of the SA-dependent marker PR1 is severely compromised in pbs3 mutants with no significant induction by 24 hpi with virulent Psm ES4326 (Fig. 2B) and an approximately 36-h delay in induction in response to avirulent Pst (avrRpt2; Fig. 2A). Pathogen-induced expression of PR1 requires the ankyrin repeat-containing master regulator NPR1 (Cao et al., 1994; Delaney et al., 1995). Expression of the SA biosynthetic gene ICS1 and PBS3 is correlated (Fig. 5C) and ICS1 and PBS3 expression precedes the dramatic induction of PR1 expression (Fig. 5A; most obvious in response to Psp and avirulent Pst). In pbs3 mutants, despite the elevated induced free SA levels at 24 hpt with Pst (avrRpt2), PR1 is not induced (Fig. 2). This result suggests that elevated free SA alone is not sufficient to activate PR1 expression.
Perhaps, at these time points, total SA (free SA + SAG) better represents plant cell exposure to free SA during the first 24 h after pathogen inoculation. Loss of PBS3 function would then lead to a reduction in PR1 expression and other defenses because it results in a reduction in total SA levels. The dramatic reduction of PR1 expression at 24 hpi with Pst (avrRpt2) compared to a 2- to 3-fold reduction in total SA levels in the pbs3 mutants can be explained if an SA threshold (best assessed as total SA) is required for induction of PR1 expression. This SA threshold is consistent with the previously proposed SA amplification loop (e.g. Jirage et al., 1999; Shah, 2003).
Review of the relevant literature indicates that when total leaf SA levels are less than 3 μg/g fresh weight, as reported here for the pbs3 mutants, PR1 induction is not typically observed (e.g. Zeier et al., 2004). In addition, when transgenic plant lines overexpressing bacterial SA biosynthetic genes exhibit constitutive expression of total SA of ≤3 μg/g fresh weight, PR1 is not significantly induced (Verberne et al., 2000; Mauch et al., 2001). High levels of SA provided by exogenous SA application would then surpass the SA threshold and restore induced PR1 expression in the pbs3-1 mutant as observed. Exogenous SA application also restores PR1 expression in mutants thought to participate in the SA amplification loop (Parker et al., 1996; Glazebrook et al., 1997; Zhou et al., 1998; Falk et al., 1999; Jirage et al., 1999; Feys et al., 2001; Shah, 2003).
An alternate explanation of our results is that SAG is the active form of SA and is required for PR1 expression. However, SAG is unlikely to be active itself because its hydrolysis is required for activation of PR1 induction (Hennig et al., 1993).
In either case, these findings suggest that PBS3 functions upstream of SA synthesis, either in a regulatory capacity (which may include an amplification loop) or in SA biosynthesis. However, this function still requires an explanation of how free SA levels could be elevated in the pbs3 mutants, whereas SAG (and total SA) is dramatically reduced. Another viable hypothesis is that SA needs to be modified by PBS3 to form SAG and to activate PR1. However, in this case, exogenous SA application should not restore wild-type SAG accumulation and expression of PR1 in pbs3-1 plants. Below, we discuss these confounding findings, as well as the uncoupling of free SA levels from induced PR1, in the context of proposed biochemical activities for PBS3.
Proposed Biochemical Function of PBS3
PBS3 is a member of the GH3 family of acyl-adenylate/thioester-forming enzymes known to conjugate amino acids to phytohormones (Staswick and Tiryaki, 2004; Staswick et al., 2005); thus, it is tempting to speculate that PBS3 may act directly on SA as an SA-amino acid synthetase to form an SA-amino acid conjugate. Alternatively, PBS3 could act on a competitive inhibitor of SA or upstream of SA biosynthesis either in a regulatory capacity or through action on a precursor of SA. As discussed earlier, very little is known about SA metabolism. Although SA synthesis appears to be plastid localized (Strawn et al., 2007), we found no predicted chloroplast transit sequence for PBS3 similar to other GH3 proteins. Thus, our expectation is that PBS3, like SAGT, acts in the cytosol, although this requires experimental confirmation.
Could PBS3 Act as an SA-Amino Acid Synthetase?
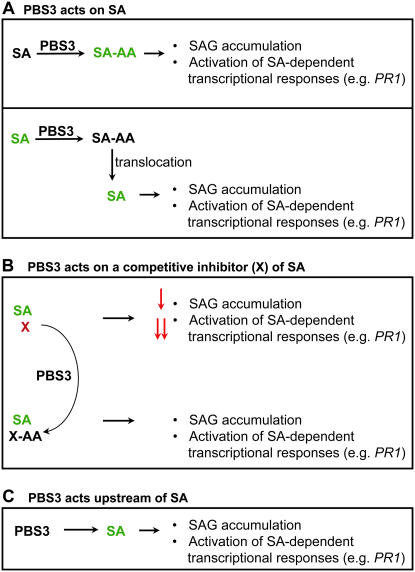
As shown in Figure 6A (top), an SA-amino acid conjugate may itself be the active form of SA required for PR1 induction. This function of PBS3 would be similar to that of JAR1, which activates JA by forming specific JA-amino acid conjugates (Staswick and Tiryaki, 2004). Although amino acid conjugates of SA have not been reported in Arabidopsis, they have been detected in grape (Vitis vinifera; Steffan et al., 1988) and bean (Phaseolus vulgaris; Bourne et al., 1991). If the SA-amino acid conjugate itself is the active form of SA required for PR1 induction, then exogenous SA application should not result in PR1 expression in the pbs3 mutant. However, we found that, at 24 hpt with SA, PR1 was expressed similarly in leaves of pbs3 and wild-type plants. This suggests that either SA-amino acid is not the active form of SA required for PR1 induction or that another GH3 family member, perhaps with lower affinity for SA, may compensate for loss of PBS3 function when supplied with a great excess of the substrate SA. One candidate for this compensatory role is GH3.5 (At4g27260), the only tested GH3 family member to exhibit adenylase activity with SA as a substrate in vitro (Staswick et al., 2002). This function of PBS3 would explain why PR1 is not induced in the pbs3 mutants despite elevated free SA levels.
Figure 6.
Proposed biochemical functions of PBS3. These models assume PBS3 activity results in conjugation of an amino acid to a small molecule (such as a phytohormone), similar to characterized GH3 family members. A, PBS3 acts directly on SA to form an SA-amino acid conjugate. In this case, SA-amino acid itself may be the active form of SA required for SAG accumulation and induction of PR1 expression (top). Alternatively, the formation of SA-amino acid may be required for a subsequent conversion to an unknown active form of SA. In the bottom panel, the formation of an SA-amino acid conjugate is required for the proper spatial localization of SA. The SA-amino acid conjugate is translocated and then hydrolyzed to release SA, resulting in SAG accumulation and PR1 induction. B, PBS3 acts on a competitive inhibitor (X) of SA to inactivate it by forming X-amino acid, resulting in full SAG accumulation and activation of SA-dependent transcriptional responses. The differential impact of X on SAG accumulation versus PR1 expression could be explained by different binding affinities of the inhibitor for distinct enzymes controlling these processes. C, PBS3 acts upstream of SA either in a regulatory capacity or as a precursor of SA. In this case, net SA biosynthesis is reduced, resulting in reduced SAG accumulation and PR1 expression. Activators of SAG accumulation and PR1 expression are shown in green, whereas inhibitors are shown in red.
Alternatively (Fig. 6A, bottom), PBS3 could act on SA to form an SA-amino acid conjugate that is required for the proper spatial localization of the active form of SA, which may be free SA. Amino acid conjugation of auxin appears to regulate its subcellular and tissue-specific distribution, and hydrolysis of phytohormone amino acid conjugates by specific amidohydrolases (e.g. IAA-amidohydrolases) allows for fine regulation of active forms of the phytohormone (Woodward and Bartel, 2005). For example, in response to Pst (avrRpt2), free SA would be synthesized in the chloroplast of a cell as an early event associated with HR. It could then be modified to SA-amino acid by PBS3 in the cytosol of the HR-undergoing cell and then exported to neighboring cells. In the neighboring cells, the SA-amino acid conjugate could be hydrolyzed, releasing free SA that could then induce PR1 expression. SAG formation catalyzed by a cytosolic SAGT could also occur in these neighboring cells. In this case, in the pbs3 mutant, free SA would be elevated because it would not be converted to SA-amino acid, and SAG formation in neighboring cells would be reduced. Exogenous SA application would negate this requirement for SA transport, thus restoring PR1 expression and SAG formation to wild-type levels in the pbs3 mutant. Consistent with this model, in tobacco, free SA accumulation preceded visible HR symptoms by >24 h, as observed using an SA reporter strain, with higher levels of free SA detected in neighboring cells surrounding HR lesions as they became apparent (Huang et al., 2006). In addition, induced PR1 accumulation was not observed in cells undergoing an HR (and subsequently dying), but specifically around HR lesions in tobacco and Arabidopsis (e.g. Dorey et al., 1997; Stone et al., 2000).
Could PBS3 Act on a Competitive Inhibitor of SA?
In this scenario, PBS3 would inactivate a competitive inhibitor X by converting it to an inactive form X-amino acid (Fig. 6B). Amino acid conjugation can either inactivate phytohormones or target them for degradation pathways (Woodward and Bartel, 2005). In pbs3 mutants, the competitive inhibitor would compete with SA in binding to the active site of enzymes that use SA as a substrate, such as SAGT. The competitive inhibitor could also bind to enzymes that are regulated by SA binding. For example, in Escherichia coli, binding of SA to the multiple antibiotic resistance repressor MarR reduces its DNA-binding affinity, thus allowing for expression of genes associated with multiple antibiotic resistance (Alekshun and Levy, 1999). In Arabidopsis, SA affects the interaction of the master regulator NPR1 (Cao et al., 1997) with TGA transcription factors enhancing DNA binding (Fan and Dong, 2002; Despres et al., 2003). If PBS3 acted on a competitive inhibitor of SA, free SA would be elevated in the pbs3 mutants and SAG and PR1 would be reduced. The differential reduction in SAG accumulation (5-fold) versus PR1 expression (approximately 50-fold) could be explained by different binding affinities of the inhibitor for distinct enzymes controlling SAG synthesis or PR1 expression. Exogenous SA (2.5 mm) would likely dominate a reversible competitive inhibitor, resulting in restored SAG and PR1 formation. The competitive inhibitor would likely be a small molecule similar to SA, such as a substituted benzoic acid. In support of this hypothesis, the Arabidopsis SAGT1 enzyme, in addition to SA, can also use benzoic acid and a subset of other substituted benzoates as substrates (Lim et al., 2002; Song, 2006). Similarly, the DNA-binding affinity of E. coli MarR can be regulated by SA and other small phenolics (Alekshun and Levy, 1999). If PBS3 inactivates an inhibitor, the reduction in total SA could then be explained by feedback inhibition on SA biosynthesis by elevated free SA.
Could PBS3 Act Upstream of SA Biosynthesis?
PBS3 may act upstream of SA biosynthesis either in a regulatory capacity or through action on a precursor of SA (Fig. 6C). For example, adenylation (and amino acid conjugation) of an SA precursor might either be required for its subsequent biochemical conversion or for its proper spatial localization. If PBS3 acted upstream of SA in either a regulatory or biosynthetic capacity, application of exogenous SA would restore SAG formation and PR1 expression in the pbs3 mutants as we observed. However, we would also expect both induced free SA and SAG levels to be reduced in the pbs3 mutants; this was not observed. One possible explanation for the observed increase in free SA with decreased SAG at 24 hpi is that lack of PBS3 function delays SA biosynthesis. If free SA is first made and then subsequently converted to SAG, it is possible that, by 24 hpi with Pst (avrRpt2), the bulk of free SA has been converted to SAG in the wild type and thus free SA levels have decreased from their maximum, whereas SAG levels are still increasing. This decrease in free SA levels as SAG continues to increase has been observed (e.g. Zhou et al., 1998). In contrast, if SA synthesis is delayed in the pbs3 mutant, free SA might be at its maximum at 24 hpi, with less of it having been converted to SAG, thereby resulting in higher free SA and lower SAG levels in the pbs3 mutant. In this scenario, the induction of PR1 expression requires some threshold of total SA (free + SAG) that was not met at 24 hpi with Pst (avrRpt2) in pbs3 mutants.
In summary, our results suggest that PBS3 plays an important role in pathogen-induced SA metabolism and that this function is critical to SAG accumulation, PR1 expression, and both basal and R-protein-mediated resistance. Given the function of GH3 family members of acyl-adenylase thioester-forming enzymes, it is likely that PBS3 acts as a small molecule-amino acid synthetase, either upstream of SA biosynthesis, on SA, or on a competitive inhibitor of SA. Further work is clearly needed to unravel the complexity of SA synthesis, activation, processing, and catabolism. In addition, our work with the pbs3-1 mutant, which contains two point mutations resulting in nonconserved amino acid changes in the C terminus of the PBS3 protein, highlights the importance of this uncharacterized region of GH3 family members and provides a framework for detailed mechanistic analyses of GH3 function.
MATERIALS AND METHODS
Bacteria, Plants, and Growth Conditions
Pseudomonas syringae strains Psm ES4326 and Pst DC3000 have been described previously (Dong et al., 1991; Whalen et al., 1991). The avirulence gene avrRpt2 was cloned into broad host-range vector pVSP61 and introduced into these strains by triparental mating as described by Kunkel et al. (1993). Bacteria were cultured on King's B medium (10 mg/mL protease peptone; 15 mg/mL glycerol; 1.5 mg/mL K2HPO4; 4 mm MgSO4, pH 7.0) supplemented with 100 μg/mL streptomycin (ES4326) or 100 μg/mL rifampicin (DC3000) plus 50 μg/mL kanamycin when avrRpt2 was present in the strain. Arabidopsis (Arabidopsis thaliana) var. Col-0 and var. No-0 were grown in Metromix 360 in growth rooms under a 9-h light/15-h dark cycle at 23°C. Isolation of the pbs3-1 mutant was described by Warren et al. (1999). T-DNA insertion lines (Col-0 genetic background) were obtained from the ABRC (Alonso et al., 2003). A Ds transposon insertion allele of PBS3 (No-0 genetic background) was obtained from the RIKEN Plant Functional Genomics Research Group (Kuromori et al., 2004).
Genetic Mapping of pbs3-1
F2 progeny of a pbs3-1 cross to Arabidopsis var. Landsberg erecta were used to genetically map the PBS3 gene. F2 plants were inoculated with Pst DC3000 (avrRpt2) and scored 3 dpi. Plants displaying a pbs3-1 phenotype were used for mapping. Initially, the pbs3-1 mutation was mapped to chromosome 5 between microsatellite markers nga249 and nga151. To further localize the PBS3, we scored approximately 800 F2 plants with these two markers and identified 70 plants with recombination events within this interval. F3 progeny of these plants were assayed for resistance to DC3000 (avrRpt2). Analysis of these lines placed the pbs3 mutation between markers MBJ5NOT and CHS1, an interval of approximately 300 kb. A collection of 40 Arabidopsis T-DNA insertion lines with insertions in genes in this interval was then obtained from the ABRC and assayed for resistance. SALK line 018225 displayed a susceptible phenotype. Location of the T-DNA insertion in this line was confirmed by PCR amplification and sequencing of the T-DNA junction fragments. A Ds insertion in the same gene was obtained from the RIKEN Plant Functional Genomics Research Group and the insertion site also confirmed by sequencing junction fragments. The pbs3-1 mutant allele was amplified by PCR and the PCR product directly sequenced. All sequencing reactions were performed using BigDye Terminator kits (Applied Biosystems) and separated on an ABI 3730 automated DNA sequencer.
Complementation of pbs3 Mutants
A full-length PBS3 genomic sequence, including the promoter region and 3′-untranslated region, was amplified from Col-0 genomic DNA using the Eppendorf TripleMaster PCR system (Eppendorf) and the following primers: 5′-CTGCAGAAATTTTGCAGAAGTTCCTT-3′ and 5′-CTGCAGTAACGAAGGGTTTGGTTTCA-3′, which contain PstI restriction sites at their 5′ ends. The PCR product was ligated into the pGEM-T Easy plasmid vector (Promega) and transformed into Escherichia coli strain DH10B. The PBS3 insert was then removed from this clone by digestion with PstI and ligated with the binary vector pGreen0229 digested with PstI (Hellens et al., 2000). This DNA sequence contains a full-length At5g13320 gene, including 1,020 bp upstream of the translation initiation site to 127 bp downstream of the stop codon.
The pGreen0229:At5g13320 construct was transformed into Agrobacterium tumefaciens strain GV3101 carrying helper plasmid pSOUP and disarmed Ti plasmid pMP90 by electroporation and selected on Luria-Bertani plates containing 50 μg/mL kanamycin sulfate (Sigma). Arabidopsis plants were transformed using the floral-dip method (Clough and Bent, 1998). Transgenic plants were selected by spraying seedlings growing in flats of Metromix with Finale herbicide (Farnam Companies) at a concentration of 0.1 g/L glufosinate ammonium (0.5 mm). T1 plants surviving herbicide selection were transplanted to pots and grown for 5 weeks, then assayed for disease phenotypes after inoculation with Psm strain ES4326.
Measurement of Bacterial Growth in Arabidopsis Leaves
Leaves of 5-week-old plants were injected with Psm ES4326 at a dose of 103 cfu/cm2 leaf area (OD600 = 0.0002). At 1 and 72 h, a 0.7-cm-diameter disc from each of 12 leaves was excised using a cork borer. These 12 discs were divided into four replicates of three leaf discs each and ground in 1 mL 10 mm MgCl2 with a plastic pestle. Appropriate dilutions were plated on King's B medium containing streptomycin and bacterial colonies were counted. Data are reported as means and sds of the log (cfu/cm2) of four replicates. Growth assays were performed twice with similar results.
Semiquantitative Scoring of Disease Phenotypes
Leaves of 5-week-old plants grown in chambers were injected with Psm ES4326 at a dose of 103 cfu/cm2 leaf area (OD600 = 0.0002). At 72 h, 10 leaves from each line were evaluated for disease symptoms and given a qualitative score: 1 = no symptoms; 2 = slight chlorosis; 3 = severe chlorosis; 4 = severe chlorosis and some necrotic lesions; 5 = leaf collapse (see Supplemental Fig. S1A). Data are reported as means and sds of the qualitative disease score. The disease assay was performed twice with similar results. A pairwise t test was used to determine whether differences between lines were significant.
Measurement of SA and SAG Levels in Arabidopsis Leaves
Arabidopsis plants for SA analysis were grown in Scotts Metro-Mix 200 with a 12-h photoperiod at a photosynthetically activated radiation of 100 to 150 μE m−2 s−1. The ethyl methanesulfonate mutant pbs3-1, the SALK T-DNA insertion line pbs3-2, eds16-1, and Col-0 were infected at 4 weeks with OD600 0.0001 Pst DC3000 containing the avirulence gene avrRpt2 on the pVSP61 plasmid (Kunkel et al., 1993). Overnight cultures of Pst DC3000 (avrRpt2) grown in King's B with rifampicin to OD600 0.7 were pelleted, resuspended in sterile 10 mm MgSO4, and diluted to OD600 0.0001. Three mature, fully expanded rosette leaves per plant were infiltrated with either OD600 0.0001 Pst in 10 mm MgSO4 or 10 mm MgSO4 as a negative control using a needleless syringe. Leaves were collected at 1 dpi, frozen in liquid nitrogen, and stored at −80°C.
The protocol for SA extraction and analysis was adapted from Dewdney et al. (2000). Frozen leaf samples (approximately 0.5 g) were ground to a powder in a prechilled mortar and pestle using liquid nitrogen. The ground leaf material was transferred to a glass tube and suspended in 3 mL of 90% MeOH. Five hundred nanograms of o-anisic acid (Aldrich) in 100% MeOH was added to each sample as an internal standard. Samples were vortexed, sonicated in a water bath sonicator for 20 min, and centrifuged at 5,000 rpm for 15 min at 4°C. The supernatant was transferred to a new tube and the brown pellet was resuspended in 2 mL 90% MeOH with vortexing. This suspension was sonicated for 20 min and centrifuged for 15 min at 4°C. The two supernatants from each sample were combined, vortexed to mix, and divided into two equal portions in new tubes (for free and total SA measurement). The solvent was evaporated using a dry vacuum at approximately 5 Torr.
For total SA, 500 μL of 80 units/mL β-glucosidase (Fluka) in 100 mm sodium acetate (pH 5.2) were added. The samples were sonicated for 5 min, vortexed, and incubated for 90 min at 37°C. For both total and free SA, 2.5 mL 5% TCA (Sigma) was added, and samples were vortexed, sonicated for 5 min, and centrifuged at 5,000 rpm for 15 min at 4°C. The supernatant was transferred to a new tube and extracted two times with 2.5 mL of a 1:1 mixture of ethyl acetate and cyclopentane. Organic phases were combined in a new tube and the solvent was evaporated under vacuum as above. The evaporated samples were stored at −80°C until ready to load on the HPLC. Prior to loading, samples were resuspended in 125 μL 20% MeOH, vortexed, sonicated for 5 min, and filtered through a 0.45-μm polytetrafluoroethylene filter (Millipore).
HPLC separation of leaf extracts was performed on a Shimadzu SCL-10A system with a Shimadzu RF-10A scanning fluorescence detector and a Shimadzu SPD-M10A photodiode array detector. Samples were separated on a 5-μm, 15 cm × 4.6-mm i.d. Supelcosil LC-ABZ Plus column (Supelco) preceded by a LC-ABZ Plus guard column maintained at 27°C. Prior to loading the 50-μL sample, the column was equilibrated with 15% acetonitrile in 25 mm KH2PO4, pH 2.5, at a flow rate of 1.0 mL/min. The concentration of acetonitrile was increased linearly to 20% over 15 min, followed by isocratic flow at 20% for 5 min, followed by a linear increase from 20% to 43% over 23 min, a linear increase from 43% to 66% over 2 min, isocratic flow at 66% for 5 min, a linear decrease from 66% to 15% over 5 min, and isocratic flow at 15% for 3 min.
o-Anisic acid and SA were quantified using a fluorescence detector set at 305-nm excitation/365-nm emission for o-anisic acid and 305/407 for SA. Calibration curves were y = 4104.6x (r2 = 0.9997) for o-anisic acid and y = 3893.8x (r2 = 0.9988) for SA, with x in nanograms and y in area units. Under these HPLC conditions, SA eluted at approximately 22 min and o-anisic acid at 10 min. The percent recovery of SA was estimated from that of o-anisic acid and ranged from 60% to 70% in three separate experiments. The detection limit for o-anisic acid and SA were approximately 0.5 ng. SAG is calculated for paired samples as total SA − free SA.
Analysis of PBS3, PR1, and ICS1 mRNA Levels
Publicly available Affymetrix Gene Chip data were accessed through the GENEVESTIGATOR Web portal (http://www.genevestigator.ethz.ch/at; Zimmermann et al., 2004). Using the digital northern tool on this site, we determined that PBS3 was expressed at low levels (typically called absent) under the vast majority of experimental conditions (data from 2,507 whole-genome ATH1 chips). Exceptions were from experiments examining biotic stress.
Two particularly informative experiments compared expression in plants infected with different bacterial strains. Expression data from PBS3 (At5g13320) and ICS1 (At1g74710) were downloaded from NASCArrays (Craigon et al., 2004). Expression in response to infection with strains of P. syringae was examined in the dataset AtGenExpress: response to the virulent, avirulent, type III secretion system deficient, and nonhost bacteria (NASCArrays-120) was performed by Thorsten Nürnberger. Expression in response to E. coli and P. syringae lacking functional flagellin or type III secretion systems was examined in the dataset: Genome-wide transcriptional analysis of the compatible Arabidopsis-P. syringae pv tomato DC3000 interaction (NASCArrays-340) was performed by William Underwood (Thilmony et al., 2006).
Correlation analyses were performed by downloading the full ATH1 expression dataset from NASCArrays (Craigon et al., 2004) and then limiting the dataset to those slides (GeneChips) using plant stock code N1092 (Col-0), a total of 821 GeneChips. The Pearson correlation (Freedman, 2005) between log2 expression values of ICS1 (At1g74710) and the remaining >22 K genes on the ATH1 GeneChip and between PBS3 (At5g13320) and the remaining genes was computed across these 821 GeneChips.
For quantitative reverse transcription PCR (qRT-PCR), RNA was isolated from infected leaf tissue immediately before inoculation and 6, 12, and 24 hpi. RNA was purified using an RNeasy plant mini kit. cDNA was generated using a high-capacity cDNA reverse transcription kit from Applied Biosystems (Fig. 2) and random primers. qRT-PCR analyses were performed using the SYBR Green PCR Mastermix kit from Applied Biosystems (Fig. 2) or the SYBR Premix Ex Taq kit from TaKaRa Bio USA (Fig. 4), and reactions were run on a Stratagene Mx3000 qRT-PCR system. Primer sequences for qRT-PCR reactions are listed in Supplemental Table S2. For all primer pairs, amplification of a single product was confirmed using melting curve analysis. Efficiency of amplification was calculated by generating a standard curve using known dilutions of a wild-type Col-0 cDNA preparation. Default parameters of the Mx3000 instrument were used for calculating threshold cycle (Ct) values for each sample (i.e. the cycle number at which the detectable fluorescence signal began to increase exponentially). Relative expression values for each sample were normalized to α-TUBULIN3 (TUA3) using the formula 2(Ct tubulin − Ct target gene). Data presented in Figure 2 are the mean of three technical repeats employing the same cDNA template. The entire experiment (inoculations, RNA extractions, cDNA synthesis, and qRT-PCR) was repeated three times with similar results. Data presented in Figure 4 are the means of three biological repeats.
For RT-PCR, RNA was isolated from infected tissue immediately before inoculation, 12, 24, and 48 hpi with Pst DC3000 (avrRpt2) OD600 = 0.0001. RNA was purified using the TRIzol method (Invitrogen), and cDNA was generated using SuperScript III (Invitrogen) with random primers. Primers used to amplify UBIQUITIN5 (UBQ5; At3g62250) were UBQ5F (5′-GTGGTGCTAAGAAGAGGAAGA-3′) and UBQ5R (5′-TCAAGCTTCAACTCCTTCTTT-3′) yielding a 250-bp product. Primers used to amplify PR1 (At2g14610) were PR1F (5′-TAGCCCACAAGATTATCTAAGG-3′) and PR1R (5′-CTCGTTCACATAATTCCCAC-3′) generating a 391-bp product. Primers used to amplify PBS3 (At5g13320) were PBS3F (5′-GAAGATGTGAAACTTGGGTGCAC-3′) and PBS3R (5′-CCTCCATTACCAAACAACACG-3′) yielding a 391-bp product. The PBS3 primers flank intron 3, the site of the T-DNA insertion in pbs3-2.
Exogenous SA Treatment
Five-week-old plants were sprayed with a 2.5 mm SA solution adjusted to pH 7.0. Leaves were collected immediately before spraying and 24 h after spraying. qRT-PCR and SA analyses were performed as described above. For bacterial growth assays following SA or INA pretreatment, either 4 mm SA or 0.65 mm of the active SA analog INA were employed. Bacterial counts were performed as detailed above.
Phylogenetic Analysis of the Arabidopsis GH3 Family
Protein sequences for the 19 Arabidopsis GH3 proteins were obtained from The Arabidopsis Information Resource and aligned using Megalign with the ClustalW method (Thompson et al., 1994) from the DNASTAR software package. Identical residues were highlighted in black and conserved residues in gray. The three groups are those determined by Staswick et al. (2002) based on phylogeny. The three motifs that form an AMP-binding domain in acyl-adenylases (Chang et al., 1997) are present in all 19 proteins.
Sequence data from this article can be found in the GenBank data library under accession numbers NM_121335 (cDNA sequence for PBS3/At5g13320) and NP_196836 (protein sequence for PBS3/At5g13320).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Semiquantitative disease-scoring scale.
Supplemental Figure S2. PBS3 transcript in pbs3-1 and pbs3-2 mutants.
Supplemental Figure S3. Protein sequence alignment of the Arabidopsis GH3 family.
Supplemental Table S1. SA levels in pbs3 mutants normalized to wild-type Col-0 in pathogen-infected leaves.
Supplemental Table S2. Primers used for qRT-PCR.
Supplementary Material
Acknowledgments
We thank the ABRC at Ohio State for providing seed of the SALK T-DNA insertion lines and the RIKEN Plant Functional Genomics Research Group for providing the Ds insertion line. We also thank Greg Hather (University of California, Berkeley; Statistics) for performing the correlation analyses.
This work was supported by the National Institutes of Health (grant no. R01 GM46451 to R.W.I.) and StartUp Funds provided by the University of California, Berkeley (to M.C.W.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: R.W. Innes (rinnes@indiana.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Alekshun MN, Levy SB (1999) The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol 7 410–413 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 [DOI] [PubMed] [Google Scholar]
- Bourne DJ, Barrow KD, Milborrow BV (1991) Salicyloylaspartate as an endogenous component in the leaves of Phaseolus vulgaris. Phytochemistry 30 4041–4044 [Google Scholar]
- Cao H, Bowling SA, Gordon S, Dong X (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88 57–63 [DOI] [PubMed] [Google Scholar]
- Chang KH, Xiang H, Dunaway-Mariano D (1997) Acyl-adenylate motif of the acyl-adenylate/thioester-forming enzyme superfamily: a site-directed mutagenesis study with the Pseudomonas sp. strain CBS3 4-chlorobenzoate:coenzyme A ligase. Biochemistry 36 15650–15659 [DOI] [PubMed] [Google Scholar]
- Chong J, Pierrel MA, Atanassova R, Werck-Reichhart D, Fritig B, Saindrenan P (2001) Free and conjugated benzoic acid in tobacco plants and cell cultures: induced accumulation upon elicitation of defense responses and role as salicylic acid precursors. Plant Physiol 125 318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Craigon DJ, James N, Okyere J, Higgins J, Jotham J, May S (2004) NASCArrays: a repository for microarray data generated by NASC's transcriptomics service. Nucleic Acids Res 32 D575–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JV, Mills JD (2004) Uptake of salicylic acid 2-O-beta-D-glucose into soybean tonoplast vesicles by an ATP-binding cassette transporter-type mechanism. Physiol Plant 120 603–612 [DOI] [PubMed] [Google Scholar]
- Dean JV, Mohammed LA, Fitzpatrick T (2005) The formation, vacuolar localization, and tonoplast transport of salicylic acid glucose conjugates in tobacco cell suspension cultures. Planta 221 287–296 [DOI] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals JA (1995) Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA 92 6602–6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewdney J, Reuber TL, Wildermuth MC, Devoto A, Cui J, Stutius LM, Drummond EP, Ausubel FM (2000) Three unique mutants of Arabidopsis identify eds loci required for limiting growth of a biotrophic fungal pathogen. Plant J 24 205–218 [DOI] [PubMed] [Google Scholar]
- Dong X, Mindrinos M, Davis KR, Ausubel FM (1991) Induction of Arabidopsis thaliana defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell 3 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey S, Baillieul F, Pierrel M-A, Saindrenan P, Fritig B, Kauffmann S (1997) Spatial and temporal induction of cell death, defense genes, and accumulation of salicylic acid in tobacco leaves reacting hypersensitively to a fungal glycoprotein elicitor. Mol Plant Microbe Interact 10 646–655 [Google Scholar]
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42 185–209 [DOI] [PubMed] [Google Scholar]
- Enyedi AJ, Raskin I (1993) Induction of UDP-glucose:salicylic acid glucosyltransferase activity in tobacco mosaic virus-inoculated tobacco (Nicotiana tabacum) leaves. Plant Physiol 101 1375–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi AJ, Yalpani N, Silverman P, Raskin I (1992) Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA 89 2480–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk A, Feys BJ, Frost LN, Jones JD, Daniels MJ, Parker JE (1999) EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci USA 96 3292–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Dong X (2002) In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. Plant Cell 14 1377–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Moisan LJ, Newman MA, Parker JE (2001) Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J 20 5400–5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DA (2005) Statistical Models: Theory and Practice. Cambridge University Press, New York
- Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 205–227 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Rogers EE, Ausubel FM (1996) Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, Holub EB, Hammerschmidt R, Ausubel FM (1997) Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 146 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick AM, Lu X, Dunaway-Mariano D (2004) Crystal structure of 4-chlorobenzoate:CoA ligase/synthetase in the unliganded and aryl substrate-bound states. Biochemistry 43 8670–8679 [DOI] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle TJ (1985) Rapid induction of selective transcription by auxins. Mol Cell Biol 5 1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDJ (1996) Resistance gene-dependent plant defense responses. Plant Cell 8 1773–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42 819–832 [DOI] [PubMed] [Google Scholar]
- Hennig J, Malamy J, Grynkiewicz G, Indulski J, Klessig DF (1993) Interconversion of the salicylic acid signal and its glucoside in tobacco. Plant J 4 593–600 [DOI] [PubMed] [Google Scholar]
- Huang WE, Huang L, Preston GM, Naylor M, Carr JP, Li Y, Singer AC, Whiteley AS, Wang H (2006) Quantitative in situ assay of salicylic acid in tobacco leaves using a genetically modified biosensor strain of Acinetobacter sp. ADP1. Plant J 46 1073–1083 [DOI] [PubMed] [Google Scholar]
- Innes RW (1998) Genetic dissection of R gene signal transduction pathways. Curr Opin Plant Biol 1 299–304 [DOI] [PubMed] [Google Scholar]
- Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J (1999) Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc Natl Acad Sci USA 96 13583–13588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDJ, Dangl JL (2006) The plant immune system. Nature 444 323–329 [DOI] [PubMed] [Google Scholar]
- Kenton P, Darby RM, Shelley G, Draper J (2000) A PR-5 promoter from Asparagus officinalis (AoPRT-L) is not induced by abiotic stress, but is activated around sites of pathogen challenge and by salicylate in transgenic tobacco. Mol Plant Pathol 1 367–378 [DOI] [PubMed] [Google Scholar]
- Klessig DF, Malamy J (1994) The salicylic acid signal in plants. Plant Mol Biol 26 1439–1458 [DOI] [PubMed] [Google Scholar]
- Kunkel BN, Bent AF, Dahlbeck D, Innes RW, Staskawicz BJ (1993) RPS2, an Arabidopsis disease resistance locus specifying recognition of Pseudomonas syringae strains expressing the avirulence gene avrRpt2. Plant Cell 5 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromori T, Hirayama T, Kiyosue Y, Takabe H, Mizukado S, Sakurai T, Akiyama K, Kamiya A, Ito T, Shinozaki K (2004) A collection of 11,800 single-copy Ds transposon insertion lines in Arabidopsis. Plant J 37 897–905 [DOI] [PubMed] [Google Scholar]
- Lee HI, Leon J, Raskin I (1995) Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA 92 4076–4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HI, Raskin I (1999) Purification, cloning, and expression of a pathogen inducible UDP-glucose:salicylic acid glucosyltransferase from tobacco. J Biol Chem 274 36637–36642 [DOI] [PubMed] [Google Scholar]
- Lim EK, Doucet CJ, Li Y, Elias L, Worrall D, Spencer SP, Ross J, Bowles DJ (2002) The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J Biol Chem 277 586–592 [DOI] [PubMed] [Google Scholar]
- Malamy J, Hennig J, Klessig DF (1992) Temperature-dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell 4 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F, Mauch-Mani B, Gaille C, Kull B, Haas D, Reimmann C (2001) Manipulation of salicylate content in Arabidopsis thaliana by the expression of an engineered bacterial salicylate synthase. Plant J 25 67–77 [DOI] [PubMed] [Google Scholar]
- Nakatsu T, Ichiyama S, Hiratake J, Saldanha A, Kobashi N, Sakata K, Kato H (2006) Structural basis for the spectral difference in luciferase bioluminescence. Nature 440 372–376 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Metraux JP (2002) EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Metraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JE, Holub EB, Frost LN, Falk A, Gunn ND, Daniels MJ (1996) Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8 2033–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Ausubel FM (1997) Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell 9 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner H-Y, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J (2003) The salicylic acid loop in plant defense. Curr Opin Plant Biol 6 365–371 [DOI] [PubMed] [Google Scholar]
- Shah J, Tsui F, Klessig DF (1997) Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant Microbe Interact 10 69–78 [DOI] [PubMed] [Google Scholar]
- Shapiro AD, Gutsche AT (2003) Capillary electrophoresis-based profiling and quantitation of total salicylic acid and related phenolics for analysis of early signaling in Arabidopsis disease resistance. Anal Biochem 320 223–233 [DOI] [PubMed] [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb C (1997) Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonich MT, Innes RW (1995) A disease resistance gene in Arabidopsis with specificity for the avrPph3 gene of Pseudomonas syringae pv phaseolicola. Mol Plant Microbe Interact 8 637–640 [DOI] [PubMed] [Google Scholar]
- Song JT (2006) Induction of a salicylic acid glucosyltransferase, AtSGT1, is an early disease response in Arabidopsis thaliana. Mol Cells 22 233–238 [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML (2002) Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15 747–754 [DOI] [PubMed] [Google Scholar]
- Steffan H, Ziegler A, Rapp A (1988) N-Salicyloyl-aspartic acid: a new phenolic compound in grapevines. Vitis 27 79–86 [Google Scholar]
- Stone JM, Heard JE, Asai T, Ausubel FM (2000) Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant (fbr) Arabidopsis mutants. Plant Cell 12 1811–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn MA, Marr SK, Inoue K, Inada N, Zubieta C, Wildermuth MC (2007) Arabidopsis isochorismate synthase functional in pathogen-induced salicylate biosynthesis exhibits properties consistent with a role in diverse stress responses. J Biol Chem 282 5919–5933 [DOI] [PubMed] [Google Scholar]
- Swiderski MR, Innes RW (2001) The Arabidopsis PBS1 resistance gene encodes a member of a novel protein kinase subfamily. Plant J 26 101–112 [DOI] [PubMed] [Google Scholar]
- Terol J, Domingo C, Talon M (2006) The GH3 family in plants: genome wide analysis in rice and evolutionary history based on EST analysis. Gene 371 279–290 [DOI] [PubMed] [Google Scholar]
- Thilmony R, Underwood W, He SY (2006) Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J 46 34–53 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero P, Merritt P, Sadanandom A, Shirasu K, Innes RW, Dangl JL (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 14 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne MC, Verpoorte R, Bol JF, Mercado-Blanco J, Linthorst HJ (2000) Overproduction of salicylic acid in plants by bacterial transgenes enhances pathogen resistance. Nat Biotechnol 18 779–783 [DOI] [PubMed] [Google Scholar]
- Warren RF, Henk A, Mowery P, Holub E, Innes RW (1998) A mutation within the leucine-rich repeat domain of the Arabidopsis disease resistance gene RPS5 partially suppresses multiple bacterial and downy mildew resistance genes. Plant Cell 10 1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RF, Merritt PM, Holub E, Innes RW (1999) Identification of three putative signal transduction genes involved in R gene-specified disease resistance in Arabidopsis. Genetics 152 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen MC, Innes RW, Bent AF, Staskawicz BJ (1991) Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 3 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414 562–565 [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J, Pink B, Mueller MJ, Berger S (2004) Light conditions influence specific defence responses in incompatible plant-pathogen interactions: uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta 219 673–683 [DOI] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J (1998) PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 10 1021–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.