Abstract
Nine different germ-line mutations in the BRCA1 breast and ovarian cancer susceptibility gene were identified in 15 of 47 kindreds from southern Sweden, by use of SSCP and heteroduplex analysis of all exons and flanking intron region and by a protein-truncation test for exon 11, followed by direct sequencing. All but one of the mutations are predicted to give rise to premature translation termination and include seven frameshift insertions or deletions, a nonsense mutation, and a splice acceptor site mutation. The remaining mutation is a missense mutation (Cys61Gly) in the zinc-binding motif. Four novel Swedish founding mutations were identified: the nucleotide 2595 deletion A was found in five families, the C 1806 T nonsense mutation in three families, the 3166 insertion TGAGA in three families, and the nucleotide 1201 deletion 11 in two families. Analysis of the intragenic polymorphism D17S855 supports common origins of the mutations. Eleven of the 15 kindreds manifesting BRCA1 mutations were breast-ovarian cancer families, several of them with a predominant ovarian cancer phenotype. The set of 32 families in which no BRCA1 alterations were detected included 1 breast-ovarian cancer kindred manifesting clear linkage to the BRCA1 region and loss of the wild-type chromosome in associated tumors. Other tumor types found in BRCA1 mutation/haplotype carriers included prostatic, pancreas, skin, and lung cancer, a malignant melanoma, an oligodendroglioma, and a carcinosarcoma. In all, 12 of 16 kindreds manifesting BRCA1 mutation or linkage contained ovarian cancer, as compared with only 6 of the remaining 31 families (P<.001). The present study confirms the involvement of BRCA1 in disease predisposition for a subset of hereditary breast cancer families often characterized by ovarian cancers.
Full text
PDF

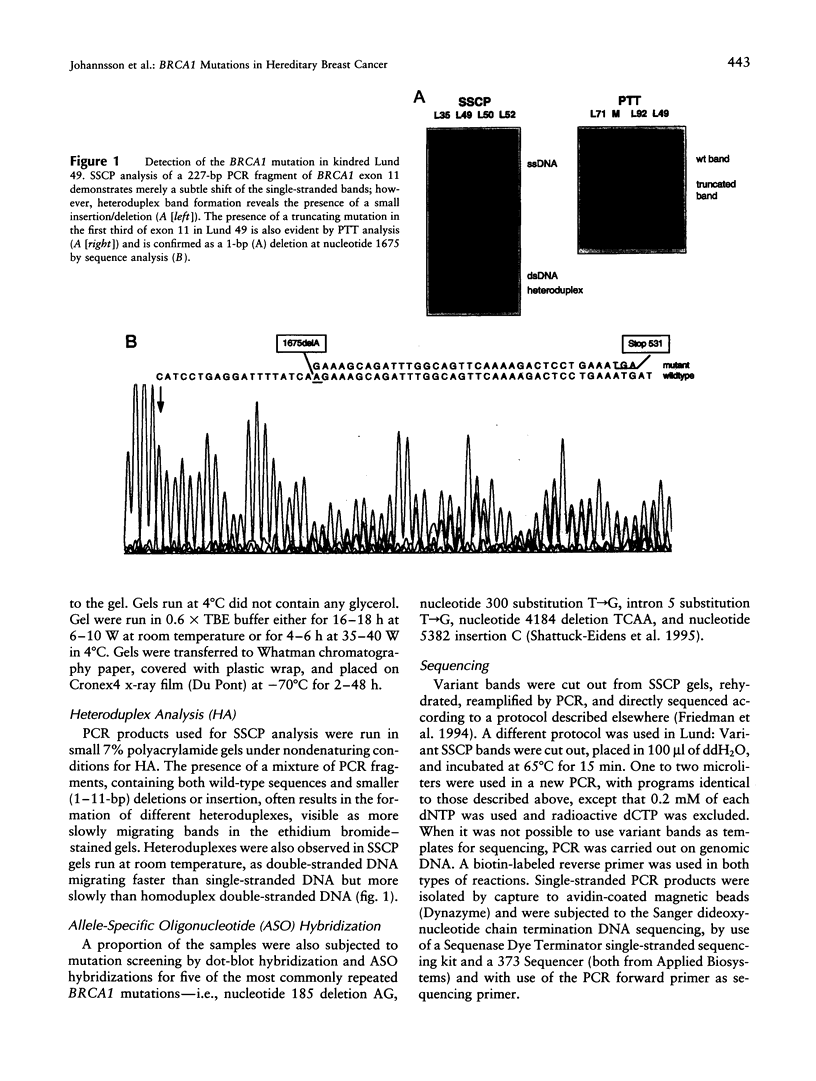







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberle H., Bierkamp C., Torchard D., Serova O., Wagner T., Natt E., Wirsching J., Heidkämper C., Montagna M., Lynch H. T. The human plakoglobin gene localizes on chromosome 17q21 and is subjected to loss of heterozygosity in breast and ovarian cancers. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6384–6388. doi: 10.1073/pnas.92.14.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. A., Nicolai H., Xu C. F., Griffiths B. L., Jones K. A., Solomon E., Hosking L., Trowsdale J., Black D. M., McFarlane R. Regulation of BRCA1. Nature. 1994 Dec 22;372(6508):733–733. doi: 10.1038/372733a0. [DOI] [PubMed] [Google Scholar]
- Campbell I. G., Nicolai H. M., Foulkes W. D., Senger G., Stamp G. W., Allan G., Boyer C., Jones K., Bast R. C., Jr, Solomon E. A novel gene encoding a B-box protein within the BRCA1 region at 17q21.1. Hum Mol Genet. 1994 Apr;3(4):589–594. doi: 10.1093/hmg/3.4.589. [DOI] [PubMed] [Google Scholar]
- Castilla L. H., Couch F. J., Erdos M. R., Hoskins K. F., Calzone K., Garber J. E., Boyd J., Lubin M. B., Deshano M. L., Brody L. C. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat Genet. 1994 Dec;8(4):387–391. doi: 10.1038/ng1294-387. [DOI] [PubMed] [Google Scholar]
- Claus E. B., Risch N., Thompson W. D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991 Feb;48(2):232–242. [PMC free article] [PubMed] [Google Scholar]
- Easton D. F., Narod S. A., Ford D., Steel M. The genetic epidemiology of BRCA1. Breast Cancer Linkage Consortium. Lancet. 1994 Sep 10;344(8924):761–761. doi: 10.1016/s0140-6736(94)92256-x. [DOI] [PubMed] [Google Scholar]
- Friedman L. S., Ostermeyer E. A., Szabo C. I., Dowd P., Lynch E. D., Rowell S. E., King M. C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994 Dec;8(4):399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- Futreal P. A., Liu Q., Shattuck-Eidens D., Cochran C., Harshman K., Tavtigian S., Bennett L. M., Haugen-Strano A., Swensen J., Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994 Oct 7;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Hogervorst F. B., Cornelis R. S., Bout M., van Vliet M., Oosterwijk J. C., Olmer R., Bakker B., Klijn J. G., Vasen H. F., Meijers-Heijboer H. Rapid detection of BRCA1 mutations by the protein truncation test. Nat Genet. 1995 Jun;10(2):208–212. doi: 10.1038/ng0695-208. [DOI] [PubMed] [Google Scholar]
- Lindblom A., Rotstein S., Nordenskjöld M., Larsson C. Linkage analysis with markers on 17q in 29 Swedish breast cancer families. Am J Hum Genet. 1993 Apr;52(4):749–753. [PMC free article] [PubMed] [Google Scholar]
- Lynch H. T., Watson P., Conway T. A., Lynch J. F. Clinical/genetic features in hereditary breast cancer. Breast Cancer Res Treat. 1990 Feb;15(2):63–71. doi: 10.1007/BF01810778. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Merajver S. D., Pham T. M., Caduff R. F., Chen M., Poy E. L., Cooney K. A., Weber B. L., Collins F. S., Johnston C., Frank T. S. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995 Apr;9(4):439–443. doi: 10.1038/ng0495-439. [DOI] [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Narod S. A., Feunteun J., Lynch H. T., Watson P., Conway T., Lynch J., Lenoir G. M. Familial breast-ovarian cancer locus on chromosome 17q12-q23. Lancet. 1991 Jul 13;338(8759):82–83. doi: 10.1016/0140-6736(91)90076-2. [DOI] [PubMed] [Google Scholar]
- Peltoketo H., Piao Y., Mannermaa A., Ponder B. A., Isomaa V., Poutanen M., Winqvist R., Vihko R. A point mutation in the putative TATA box, detected in nondiseased individuals and patients with hereditary breast cancer, decreases promoter activity of the 17 beta-hydroxysteroid dehydrogenase type 1 gene 2 (EDH17B2) in vitro. Genomics. 1994 Sep 1;23(1):250–252. doi: 10.1006/geno.1994.1487. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Petersen G. M., Krush A. J., Booker S., Jen J., Giardiello F. M., Hamilton S. R., Vogelstein B., Kinzler K. W. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993 Dec 30;329(27):1982–1987. doi: 10.1056/NEJM199312303292702. [DOI] [PubMed] [Google Scholar]
- Saito H., Inazawa J., Saito S., Kasumi F., Koi S., Sagae S., Kudo R., Saito J., Noda K., Nakamura Y. Detailed deletion mapping of chromosome 17q in ovarian and breast cancers: 2-cM region on 17q21.3 often and commonly deleted in tumors. Cancer Res. 1993 Jul 15;53(14):3382–3385. [PubMed] [Google Scholar]
- Schildkraut J. M., Collins N. K., Dent G. A., Tucker J. A., Barrett J. C., Berchuck A., Boyd J. Loss of heterozygosity on chromosome 17q11-21 in cancers of women who have both breast and ovarian cancer. Am J Obstet Gynecol. 1995 Mar;172(3):908–913. doi: 10.1016/0002-9378(95)90020-9. [DOI] [PubMed] [Google Scholar]
- Shattuck-Eidens D., McClure M., Simard J., Labrie F., Narod S., Couch F., Hoskins K., Weber B., Castilla L., Erdos M. A collaborative survey of 80 mutations in the BRCA1 breast and ovarian cancer susceptibility gene. Implications for presymptomatic testing and screening. JAMA. 1995 Feb 15;273(7):535–541. [PubMed] [Google Scholar]
- Simard J., Tonin P., Durocher F., Morgan K., Rommens J., Gingras S., Samson C., Leblanc J. F., Bélanger C., Dion F. Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet. 1994 Dec;8(4):392–398. doi: 10.1038/ng1294-392. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Easton D. F., Evans D. G., Ponder B. A. Allele losses in the region 17q12-21 in familial breast and ovarian cancer involve the wild-type chromosome. Nat Genet. 1992 Oct;2(2):128–131. doi: 10.1038/ng1092-128. [DOI] [PubMed] [Google Scholar]
- Struewing J. P., Abeliovich D., Peretz T., Avishai N., Kaback M. M., Collins F. S., Brody L. C. The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet. 1995 Oct;11(2):198–200. doi: 10.1038/ng1095-198. [DOI] [PubMed] [Google Scholar]
- Struewing J. P., Brody L. C., Erdos M. R., Kase R. G., Giambarresi T. R., Smith S. A., Collins F. S., Tucker M. A. Detection of eight BRCA1 mutations in 10 breast/ovarian cancer families, including 1 family with male breast cancer. Am J Hum Genet. 1995 Jul;57(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Thompson M. E., Jensen R. A., Obermiller P. S., Page D. L., Holt J. T. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995 Apr;9(4):444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- Wooster R., Neuhausen S. L., Mangion J., Quirk Y., Ford D., Collins N., Nguyen K., Seal S., Tran T., Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994 Sep 30;265(5181):2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]




