Abstract
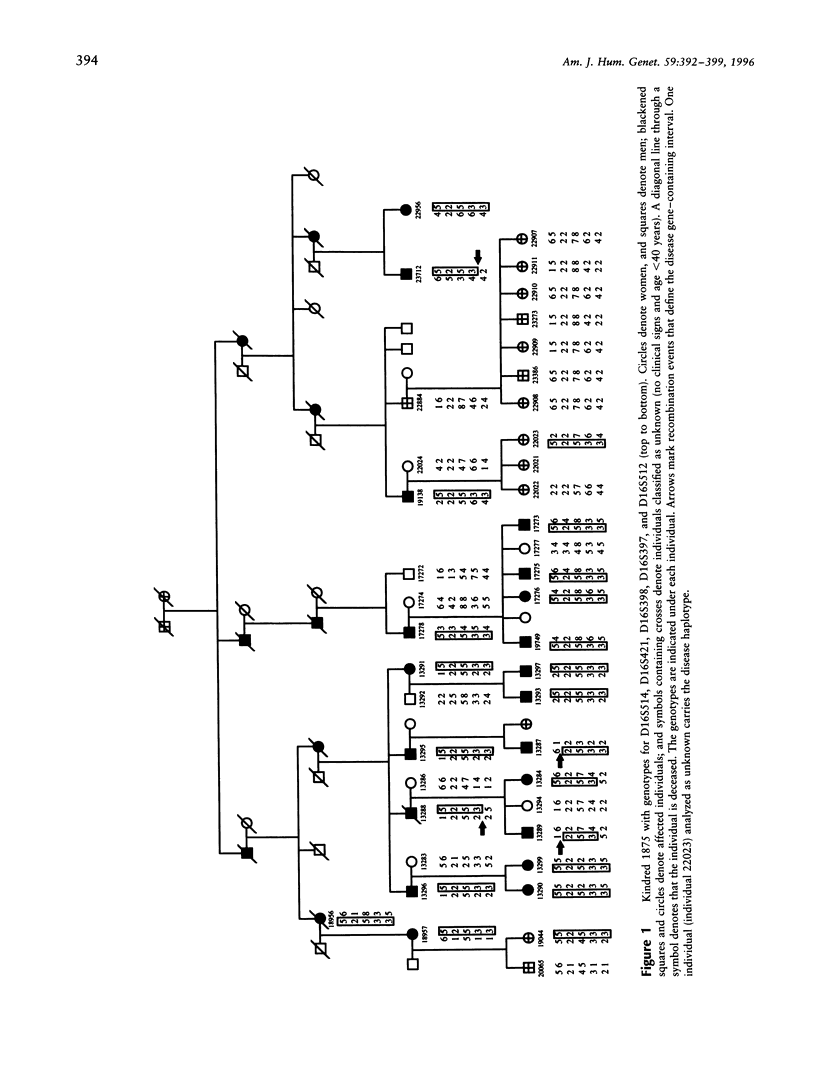
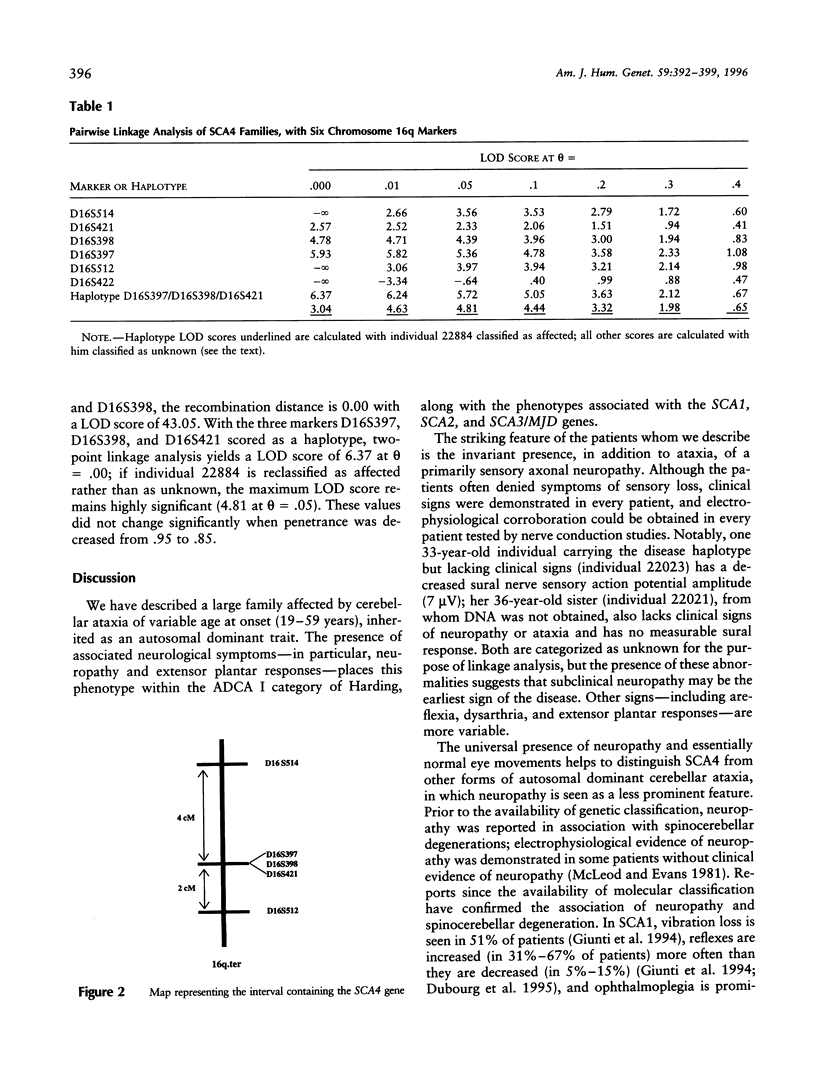
The hereditary ataxias represent a clinically and genetically heterogeneous group of neurodegenerative disorders. Various classification schemes based on clinical criteria are being replaced as molecular characterization of the ataxias proceeds; so far, seven distinct autosomal dominant hereditary ataxias have been genetically mapped in the human genome. We report linkage to chromosome 16q22.1 for one of these genes (SCA4) in a five-generation family with an autosomal dominant, late-onset spinocerebellar ataxia; the gene is tightly linked to the microsatellite marker D16S397 (LOD score = 5.93 at theta = .00). In addition, we present clinical and electrophysiological data regarding the distinct and previously unreported phenotype consisting of ataxia with the invariant presence of a prominent axonal sensory neuropathy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auburger G., Diaz G. O., Capote R. F., Sanchez S. G., Perez M. P., del Cueto M. E., Meneses M. G., Farrall M., Williamson R., Chamberlain S. Autosomal dominant ataxia: genetic evidence for locus heterogeneity from a Cuban founder-effect population. Am J Hum Genet. 1990 Jun;46(6):1163–1177. [PMC free article] [PubMed] [Google Scholar]
- BIEMOND A. La forme radiculo-cordonnale postérieure des dégénérescences spino-cérébelleuses. Rev Neurol (Paris) 1954;91(1):3–21. [PubMed] [Google Scholar]
- Banfi S., Servadio A., Chung M. Y., Kwiatkowski T. J., Jr, McCall A. E., Duvick L. A., Shen Y., Roth E. J., Orr H. T., Zoghbi H. Y. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat Genet. 1994 Aug;7(4):513–520. doi: 10.1038/ng0894-513. [DOI] [PubMed] [Google Scholar]
- Benomar A., Krols L., Stevanin G., Cancel G., LeGuern E., David G., Ouhabi H., Martin J. J., Dürr A., Zaim A. The gene for autosomal dominant cerebellar ataxia with pigmentary macular dystrophy maps to chromosome 3p12-p21.1. Nat Genet. 1995 May;10(1):84–88. doi: 10.1038/ng0595-84. [DOI] [PubMed] [Google Scholar]
- Campuzano V., Montermini L., Moltò M. D., Pianese L., Cossée M., Cavalcanti F., Monros E., Rodius F., Duclos F., Monticelli A. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996 Mar 8;271(5254):1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- Coutinho P., Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978 Jul;28(7):703–709. doi: 10.1212/wnl.28.7.703. [DOI] [PubMed] [Google Scholar]
- Dubourg O., Dürr A., Cancel G., Stevanin G., Chneiweiss H., Penet C., Agid Y., Brice A. Analysis of the SCA1 CAG repeat in a large number of families with dominant ataxia: clinical and molecular correlations. Ann Neurol. 1995 Feb;37(2):176–180. doi: 10.1002/ana.410370207. [DOI] [PubMed] [Google Scholar]
- Gispert S., Twells R., Orozco G., Brice A., Weber J., Heredero L., Scheufler K., Riley B., Allotey R., Nothers C. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1. Nat Genet. 1993 Jul;4(3):295–299. doi: 10.1038/ng0793-295. [DOI] [PubMed] [Google Scholar]
- Giunti P., Sweeney M. G., Spadaro M., Jodice C., Novelletto A., Malaspina P., Frontali M., Harding A. E. The trinucleotide repeat expansion on chromosome 6p (SCA1) in autosomal dominant cerebellar ataxias. Brain. 1994 Aug;117(Pt 4):645–649. doi: 10.1093/brain/117.4.645. [DOI] [PubMed] [Google Scholar]
- Gotoda T., Arita M., Arai H., Inoue K., Yokota T., Fukuo Y., Yazaki Y., Yamada N. Adult-onset spinocerebellar dysfunction caused by a mutation in the gene for the alpha-tocopherol-transfer protein. N Engl J Med. 1995 Nov 16;333(20):1313–1318. doi: 10.1056/NEJM199511163332003. [DOI] [PubMed] [Google Scholar]
- Gouw L. G., Kaplan C. D., Haines J. H., Digre K. B., Rutledge S. L., Matilla A., Leppert M., Zoghbi H. Y., Ptácek L. J. Retinal degeneration characterizes a spinocerebellar ataxia mapping to chromosome 3p. Nat Genet. 1995 May;10(1):89–93. doi: 10.1038/ng0595-89. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Junck L., Fink J. F. Machado-Joseph disease and SCA3: the genotype meets the phenotypes. Neurology. 1996 Jan;46(1):4–8. doi: 10.1212/wnl.46.1.4. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., Kawakami H., Nakamura S., Nishimura M., Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994 Nov;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Klanke C. A., Su Y. R., Callen D. F., Wang Z., Meneton P., Baird N., Kandasamy R. A., Orlowski J., Otterud B. E., Leppert M. Molecular cloning and physical and genetic mapping of a novel human Na+/H+ exchanger (NHE5/SLC9A5) to chromosome 16q22.1. Genomics. 1995 Feb 10;25(3):615–622. doi: 10.1016/0888-7543(95)80002-4. [DOI] [PubMed] [Google Scholar]
- Koide R., Ikeuchi T., Onodera O., Tanaka H., Igarashi S., Endo K., Takahashi H., Kondo R., Ishikawa A., Hayashi T. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Jan;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- Konigsmark B. W., Weiner L. P. The olivopontocerebellar atrophies: a review. Medicine (Baltimore) 1970 May;49(3):227–241. doi: 10.1097/00005792-197005000-00003. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet. 1985 May;37(3):482–498. [PMC free article] [PubMed] [Google Scholar]
- Matilla T., McCall A., Subramony S. H., Zoghbi H. Y. Molecular and clinical correlations in spinocerebellar ataxia type 3 and Machado-Joseph disease. Ann Neurol. 1995 Jul;38(1):68–72. doi: 10.1002/ana.410380113. [DOI] [PubMed] [Google Scholar]
- McLeod J. G., Evans W. A. Peripheral neuropathy in spinocerebellar degenerations. Muscle Nerve. 1981 Jan-Feb;4(1):51–61. doi: 10.1002/mus.880040110. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Yanagisawa H., Sato K., Shirayama T., Ohsaki E., Bundo M., Takeda T., Tadokoro K., Kondo I., Murayama N. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet. 1994 Jan;6(1):14–18. doi: 10.1038/ng0194-14. [DOI] [PubMed] [Google Scholar]
- Orozco Diaz G., Nodarse Fleites A., Cordovés Sagaz R., Auburger G. Autosomal dominant cerebellar ataxia: clinical analysis of 263 patients from a homogeneous population in Holguín, Cuba. Neurology. 1990 Sep;40(9):1369–1375. doi: 10.1212/wnl.40.9.1369. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Ouahchi K., Arita M., Kayden H., Hentati F., Ben Hamida M., Sokol R., Arai H., Inoue K., Mandel J. L., Koenig M. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat Genet. 1995 Feb;9(2):141–145. doi: 10.1038/ng0295-141. [DOI] [PubMed] [Google Scholar]
- Ptacek L. J., Tyler F., Trimmer J. S., Agnew W. S., Leppert M. Analysis in a large hyperkalemic periodic paralysis pedigree supports tight linkage to a sodium channel locus. Am J Hum Genet. 1991 Aug;49(2):378–382. [PMC free article] [PubMed] [Google Scholar]
- Ranum L. P., Duvick L. A., Rich S. S., Schut L. J., Litt M., Orr H. T. Localization of the autosomal dominant HLA-linked spinocerebellar ataxia (SCA1) locus, in two kindreds, within an 8-cM subregion of chromosome 6p. Am J Hum Genet. 1991 Jul;49(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- Ranum L. P., Schut L. J., Lundgren J. K., Orr H. T., Livingston D. M. Spinocerebellar ataxia type 5 in a family descended from the grandparents of President Lincoln maps to chromosome 11. Nat Genet. 1994 Nov;8(3):280–284. doi: 10.1038/ng1194-280. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. N. Autosomal dominant cerebellar phenotypes: the genotype has settled the issue. Neurology. 1995 Jan;45(1):1–5. doi: 10.1212/wnl.45.1.1. [DOI] [PubMed] [Google Scholar]
- Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D. A., Smith S., Uziel T., Sfez S. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995 Jun 23;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Servadio A., Koshy B., Armstrong D., Antalffy B., Orr H. T., Zoghbi H. Y. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat Genet. 1995 May;10(1):94–98. doi: 10.1038/ng0595-94. [DOI] [PubMed] [Google Scholar]
- Wadia N. H., Swami R. K. A new form of heredo-familial spinocerebellar degeneration with slow eye movements (nine families). Brain. 1971;94(2):359–374. doi: 10.1093/brain/94.2.359. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y., Jodice C., Sandkuijl L. A., Kwiatkowski T. J., Jr, McCall A. E., Huntoon S. A., Lulli P., Spadaro M., Litt M., Cann H. M. The gene for autosomal dominant spinocerebellar ataxia (SCA1) maps telomeric to the HLA complex and is closely linked to the D6S89 locus in three large kindreds. Am J Hum Genet. 1991 Jul;49(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Zoghbi H. Y., Pollack M. S., Lyons L. A., Ferrell R. E., Daiger S. P., Beaudet A. L. Spinocerebellar ataxia: variable age of onset and linkage to human leukocyte antigen in a large kindred. Ann Neurol. 1988 Jun;23(6):580–584. doi: 10.1002/ana.410230609. [DOI] [PubMed] [Google Scholar]


