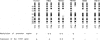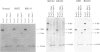Abstract
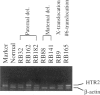
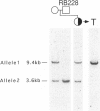
Epidemiological and genetic studies of retinoblastoma (RB) suggested that imprinted genes might be genetically linked to the RB gene. In this study, we found that the human serotonin-receptor, HTR2, gene, which had been mapped nearby the RB gene on chromosome 13, was expressed only in human fibroblasts with a maternal allele and not in cells without a maternal allele. The 5' genomic region of the human HTR2 gene was cloned by PCR-mediated method. Only the 5' region of the gene was methylated in cells with the maternal gene, and it was not methylated in cells without the maternal gene. A polymorphism of PvuII site of the gene was also found and useful for the segregation analysis in a family of a RB patient and for analysis of loss of heterozygosity on chromosome 13 in tumor and its parental origin. These results suggest that the human HTR2 gene might be affected by genomic imprinting and that exclusive expression of the maternal HTR2 gene may be associated with the delayed occurrence of RB, which had lost the maternal chromosome 13.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S., Smallwood A. A chromatin model of IGF2/H19 imprinting. Nat Genet. 1995 Nov;11(3):237–238. doi: 10.1038/ng1195-237. [DOI] [PubMed] [Google Scholar]
- Barlow D. P. Gametic imprinting in mammals. Science. 1995 Dec 8;270(5242):1610–1613. doi: 10.1126/science.270.5242.1610. [DOI] [PubMed] [Google Scholar]
- Bowcock A. Report of the First International Workshop on Human Chromosome 13 Mapping. Dallas, Texas, September 21-22, 1992. Cytogenet Cell Genet. 1993;62(2-3):89–107. doi: 10.1159/000133450. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Beechey C. V. Autosomal and X-chromosome imprinting. Dev Suppl. 1990:63–72. [PubMed] [Google Scholar]
- Cavenee W., Leach R., Mohandas T., Pearson P., White R. Isolation and regional localization of DNA segments revealing polymorphic loci from human chromosome 13. Am J Hum Genet. 1984 Jan;36(1):10–24. [PMC free article] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989 Jan 12;337(6203):138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Mukai S., Petersen R., Rapaport J. M., Walton D., Yandell D. W. Parental origin of mutations of the retinoblastoma gene. Nature. 1989 Jun 15;339(6225):556–558. doi: 10.1038/339556a0. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Weichselbaum R., Bruns G. A. Chromosome 13 restriction fragment length polymorphisms. Hum Genet. 1984;65(4):320–324. doi: 10.1007/BF00291555. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Zhu X., Becker A., Gallie B. L. Mutations in the RB1 gene and their effects on transcription. Mol Cell Biol. 1989 Nov;9(11):4596–4604. doi: 10.1128/mcb.9.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
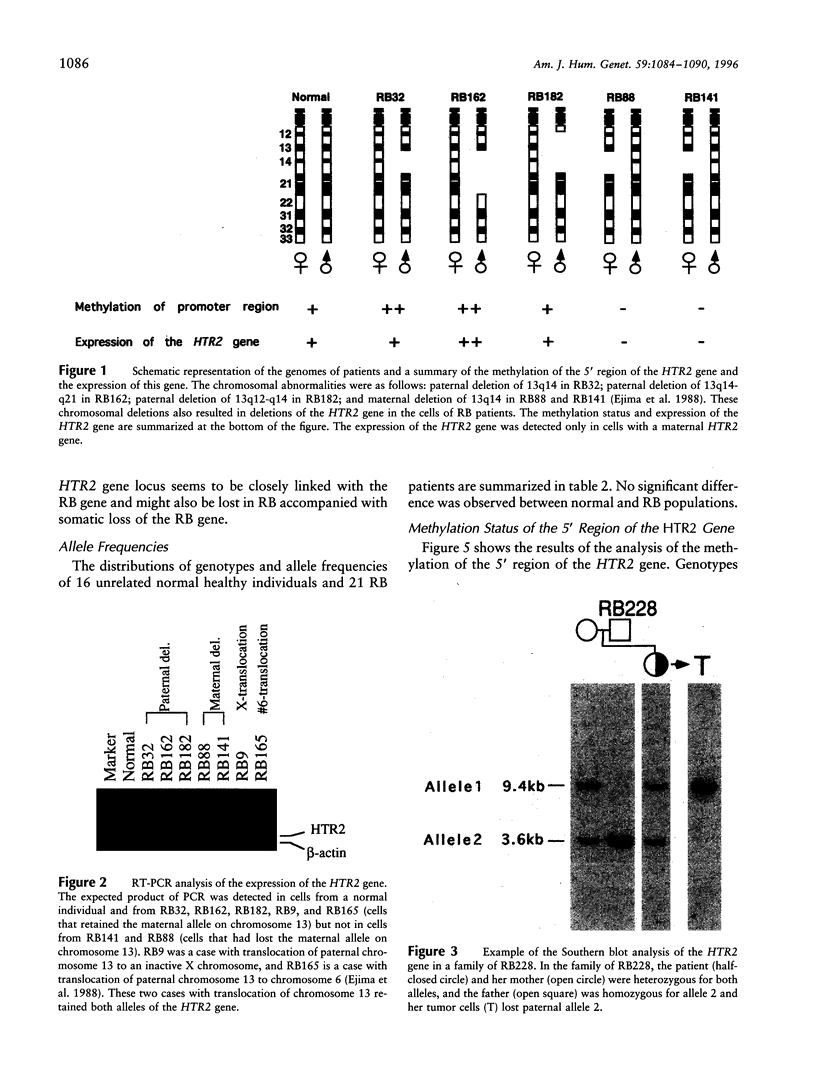
- Ejima Y., Sasaki M. S., Kaneko A., Tanooka H. Types, rates, origin and expressivity of chromosome mutations involving 13q14 in retinoblastoma patients. Hum Genet. 1988 Jun;79(2):118–123. doi: 10.1007/BF00280548. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Harrison D. J., Hooper M. L., Armstrong J. F., Clarke A. R. Effects of heterozygosity for the Rb-1t19neo allele in the mouse. Oncogene. 1995 Apr 20;10(8):1615–1620. [PubMed] [Google Scholar]
- Hsieh C. L., Bowcock A. M., Farrer L. A., Hebert J. M., Huang K. N., Cavalli-Sforza L. L., Julius D., Francke U. The serotonin receptor subtype 2 locus HTR2 is on human chromosome 13 near genes for esterase D and retinoblastoma-1 and on mouse chromosome 14. Somat Cell Mol Genet. 1990 Nov;16(6):567–574. doi: 10.1007/BF01233097. [DOI] [PubMed] [Google Scholar]
- Julius D., Huang K. N., Livelli T. J., Axel R., Jessell T. M. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci U S A. 1990 Feb;87(3):928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
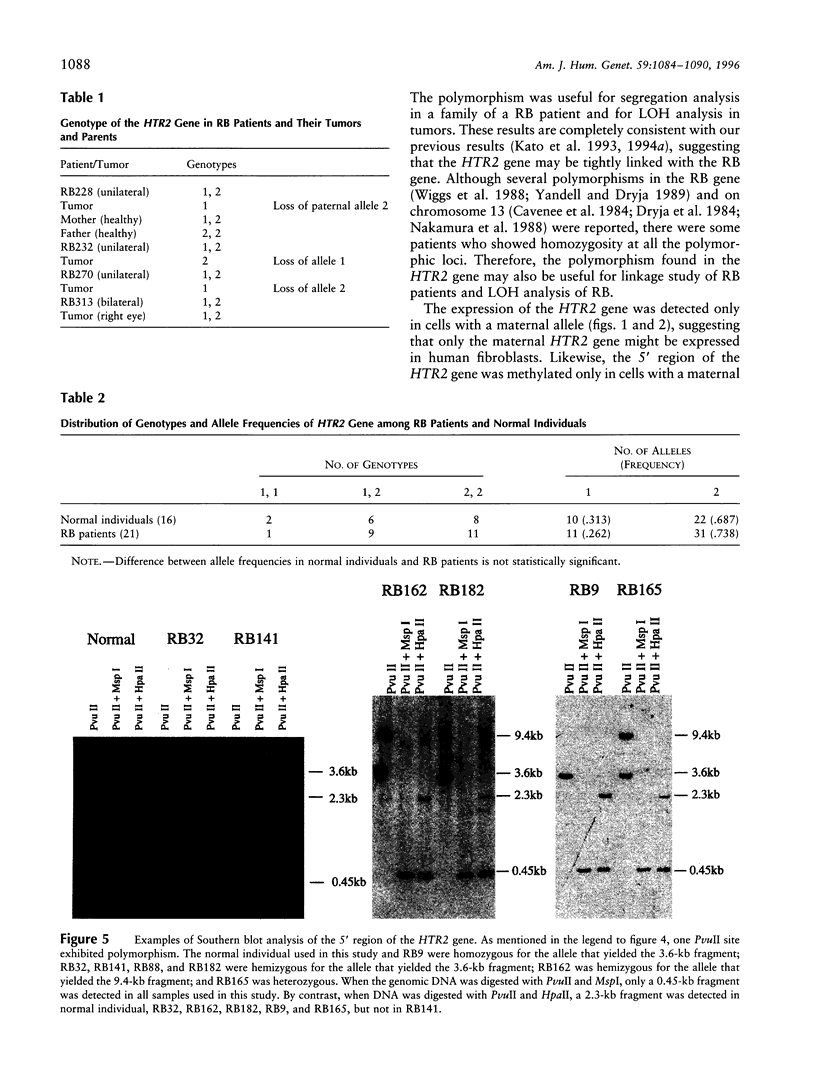
- Kato M. V., Ishizaki K., Ejima Y., Kaneko A., Tanooka H., Sasaki M. S. Loss of heterozygosity on chromosome 13 and its association with delayed growth of retinoblastoma. Int J Cancer. 1993 Jul 30;54(6):922–926. doi: 10.1002/ijc.2910540609. [DOI] [PubMed] [Google Scholar]
- Kato M. V., Ishizaki K., Shimizu T., Ejima Y., Tanooka H., Takayama J., Kaneko A., Toguchida J., Sasaki M. S. Parental origin of germ-line and somatic mutations in the retinoblastoma gene. Hum Genet. 1994 Jul;94(1):31–38. doi: 10.1007/BF02272838. [DOI] [PubMed] [Google Scholar]
- Kato M. V., Ishizaki K., Shimizu T., Toguchida J., Kaneko A., Sasaki M. S. Delayed development of retinoblastoma associated with loss of a maternal allele on chromosome 13. Int J Cancer. 1995 Feb 20;64(1):3–8. doi: 10.1002/ijc.2910640103. [DOI] [PubMed] [Google Scholar]
- Kato M. V., Ishizaki K., Toguchida J., Kaneko A., Takayama J., Tanooka H., Kato T., Shimizu T., Sasaki M. S. Mutations in the retinoblastoma gene and their expression in somatic and tumor cells of patients with hereditary retinoblastoma. Hum Mutat. 1994;3(1):44–51. doi: 10.1002/humu.1380030108. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Liu J., Chen Y., Kozak C. A., Yu L. The 5-HT2 serotonin receptor gene Htr-2 is tightly linked to Es-10 on mouse chromosome 14. Genomics. 1991 Sep;11(1):231–234. doi: 10.1016/0888-7543(91)90127-z. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Carlson M., Krapcho K., Kanamori M., White R. New approach for isolation of VNTR markers. Am J Hum Genet. 1988 Dec;43(6):854–859. [PMC free article] [PubMed] [Google Scholar]
- Naumova A., Sapienza C. The genetics of retinoblastoma, revisited. Am J Hum Genet. 1994 Feb;54(2):264–273. [PMC free article] [PubMed] [Google Scholar]
- Sekido R., Murai K., Funahashi J., Kamachi Y., Fujisawa-Sehara A., Nabeshima Y., Kondoh H. The delta-crystallin enhancer-binding protein delta EF1 is a repressor of E2-box-mediated gene activation. Mol Cell Biol. 1994 Sep;14(9):5692–5700. doi: 10.1128/mcb.14.9.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert P. D., Chenchik A., Kellogg D. E., Lukyanov K. A., Lukyanov S. A. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 1995 Mar 25;23(6):1087–1088. doi: 10.1093/nar/23.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes R. S., Lan N., Klisak I., Mohandas T., Diep A., Kojis T., Heinzmann C., Shih J. C. Assignment of a serotonin 5HT-2 receptor gene (HTR2) to human chromosome 13q14-q21 and mouse chromosome 14. Genomics. 1991 Mar;9(3):461–465. doi: 10.1016/0888-7543(91)90411-7. [DOI] [PubMed] [Google Scholar]
- Stanojević D., Hoey T., Levine M. Sequence-specific DNA-binding activities of the gap proteins encoded by hunchback and Krüppel in Drosophila. Nature. 1989 Sep 28;341(6240):331–335. doi: 10.1038/341331a0. [DOI] [PubMed] [Google Scholar]
- Toguchida J., Ishizaki K., Sasaki M. S., Nakamura Y., Ikenaga M., Kato M., Sugimot M., Kotoura Y., Yamamuro T. Preferential mutation of paternally derived RB gene as the initial event in sporadic osteosarcoma. Nature. 1989 Mar 9;338(6211):156–158. doi: 10.1038/338156a0. [DOI] [PubMed] [Google Scholar]
- Vogel F., Rathenberg R. Spontaneous mutation in man. Adv Hum Genet. 1975;5:223–318. doi: 10.1007/978-1-4615-9068-2_4. [DOI] [PubMed] [Google Scholar]
- Wiggs J., Nordenskjöld M., Yandell D., Rapaport J., Grondin V., Janson M., Werelius B., Petersen R., Craft A., Riedel K. Prediction of the risk of hereditary retinoblastoma, using DNA polymorphisms within the retinoblastoma gene. N Engl J Med. 1988 Jan 21;318(3):151–157. doi: 10.1056/NEJM198801213180305. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Dryja T. P. Detection of DNA sequence polymorphisms by enzymatic amplification and direct genomic sequencing. Am J Hum Genet. 1989 Oct;45(4):547–555. [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Dunn J. M., Goddard A. D., Squire J. A., Becker A., Phillips R. A., Gallie B. L. Mechanisms of loss of heterozygosity in retinoblastoma. Cytogenet Cell Genet. 1992;59(4):248–252. doi: 10.1159/000133261. [DOI] [PubMed] [Google Scholar]
- de Jong R., van der Heijden J., Meijlink F. DNA-binding specificity of the S8 homeodomain. Nucleic Acids Res. 1993 Oct 11;21(20):4711–4720. doi: 10.1093/nar/21.20.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]