Abstract
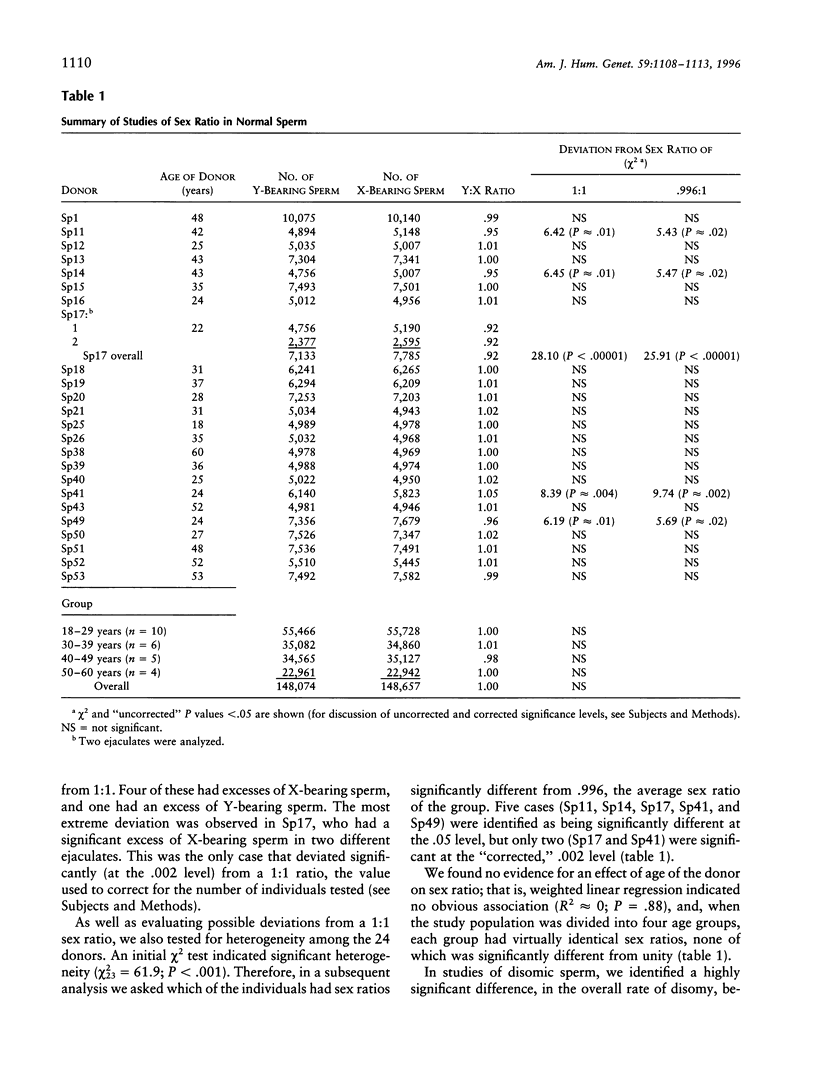
In humans, deviations from a 1:1 male:female ratio have been identified in both chromosomally normal and trisomic live births: among normal newborns there is a slight excess of males, among trisomy 18 live borns a large excess of females, and among trisomy 21 live borns an excess of males. These differences could arise from differential production of or fertilization by Y- or X-bearing sperm or from selection against male or female conceptions. To examine the proportion of Y- and X-bearing sperm in normal sperm and in sperm disomic for chromosomes 18 or 21, we used three-color FISH (to the X and Y and either chromosome 18 or chromosome 21) to analyze >300,000 sperm from 24 men. In apparently normal sperm, the sex ratio was nearly 1:1 (148,074 Y-bearing to 148,657 X-bearing sperm), and the value was not affected by the age of the donor. Certain of the donors, however, had significant excesses of Y- or X-bearing sperm. In disomy 18 sperm, there were virtually identical numbers of Y- and X-bearing sperm; thus, the excess of females in trisomy 18 presumably is due to selection against male trisomic conceptions. In contrast, we observed 69 Y-bearing and 44 X-bearing sperm disomic for chromosome 21. This is consistent with previous molecular studies, which have identified an excess of males among paternally derived cases of trisomy 21, and suggests that some of the excess of males among Down syndrome individuals is attributable to a nondisjunctional mechanism in which the extra chromosome 21 preferentially segregates with the Y chromosome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chayko C. A., Martin-DeLeon P. A. The murine Rb(6.16) translocation: alterations in the proportion of alternate sperm segregants effecting fertilization in vitro and in vivo. Hum Genet. 1992 Sep-Oct;90(1-2):79–85. doi: 10.1007/BF00210748. [DOI] [PubMed] [Google Scholar]
- Fisher J. M., Harvey J. F., Morton N. E., Jacobs P. A. Trisomy 18: studies of the parent and cell division of origin and the effect of aberrant recombination on nondisjunction. Am J Hum Genet. 1995 Mar;56(3):669–675. [PMC free article] [PubMed] [Google Scholar]
- Griffin D. K., Abruzzo M. A., Millie E. A., Sheean L. A., Feingold E., Sherman S. L., Hassold T. J. Non-disjunction in human sperm: evidence for an effect of increasing paternal age. Hum Mol Genet. 1995 Dec;4(12):2227–2232. doi: 10.1093/hmg/4.12.2227. [DOI] [PubMed] [Google Scholar]
- Hassold T., Quillen S. D., Yamane J. A. Sex ratio in spontaneous abortions. Ann Hum Genet. 1983 Jan;47(Pt 1):39–47. doi: 10.1111/j.1469-1809.1983.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Theurkauf W. E. Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet. 1993 Sep;9(9):310–317. doi: 10.1016/0168-9525(93)90249-h. [DOI] [PubMed] [Google Scholar]
- James W. H. Gonadotrophin and the human secondary sex ratio. Br Med J. 1980 Sep 13;281(6242):711–712. doi: 10.1136/bmj.281.6242.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. H., Rostron J. Parental age, parity and sex ratio in births in England and Wales, 1968-77. J Biosoc Sci. 1985 Jan;17(1):47–56. doi: 10.1017/s0021932000015467. [DOI] [PubMed] [Google Scholar]
- Lyttle T. W. Cheaters sometimes prosper: distortion of mendelian segregation by meiotic drive. Trends Genet. 1993 Jun;9(6):205–210. doi: 10.1016/0168-9525(93)90120-7. [DOI] [PubMed] [Google Scholar]
- Manoukian S., Lalatta F., Selicorni A., Tadini G., Cavalli R., Neri G. Cardio-facio-cutaneous (CFC) syndrome: report of an adult without mental retardation. Am J Med Genet. 1996 May 17;63(2):382–385. doi: 10.1002/(SICI)1096-8628(19960517)63:2<382::AID-AJMG11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Martin R. H., Spriggs E., Ko E., Rademaker A. W. The relationship between paternal age, sex ratios, and aneuploidy frequencies in human sperm, as assessed by multicolor FISH. Am J Hum Genet. 1995 Dec;57(6):1395–1399. [PMC free article] [PubMed] [Google Scholar]
- Petersen M. B., Antonarakis S. E., Hassold T. J., Freeman S. B., Sherman S. L., Avramopoulos D., Mikkelsen M. Paternal nondisjunction in trisomy 21: excess of male patients. Hum Mol Genet. 1993 Oct;2(10):1691–1695. doi: 10.1093/hmg/2.10.1691. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Petersen M. B., Freeman S. B., Hersey J., Pettay D., Taft L., Frantzen M., Mikkelsen M., Hassold T. J. Non-disjunction of chromosome 21 in maternal meiosis I: evidence for a maternal age-dependent mechanism involving reduced recombination. Hum Mol Genet. 1994 Sep;3(9):1529–1535. doi: 10.1093/hmg/3.9.1529. [DOI] [PubMed] [Google Scholar]
- Spriggs E. L., Rademaker A. W., Martin R. H. Aneuploidy in human sperm: the use of multicolor FISH to test various theories of nondisjunction. Am J Hum Genet. 1996 Feb;58(2):356–362. [PMC free article] [PubMed] [Google Scholar]
- Ulizzi L., Zonta L. A. Sex ratio and selection by early mortality in humans: fifty-year analysis in different ethnic groups. Hum Biol. 1994 Dec;66(6):1037–1048. [PubMed] [Google Scholar]
- Visaria P. M. Sex ratio at birth in territories with a relatively complete registration. Eugen Q. 1967 Jun;14(2):132–142. doi: 10.1080/19485565.1967.9987713. [DOI] [PubMed] [Google Scholar]


