Abstract
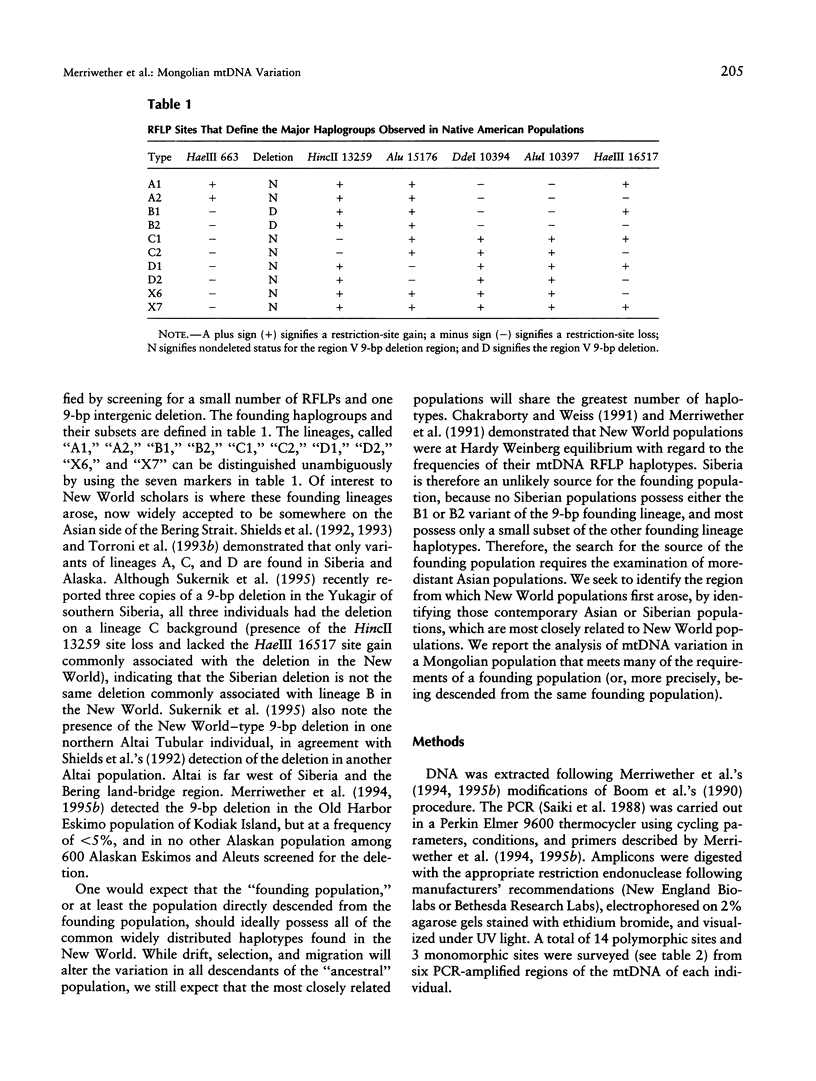
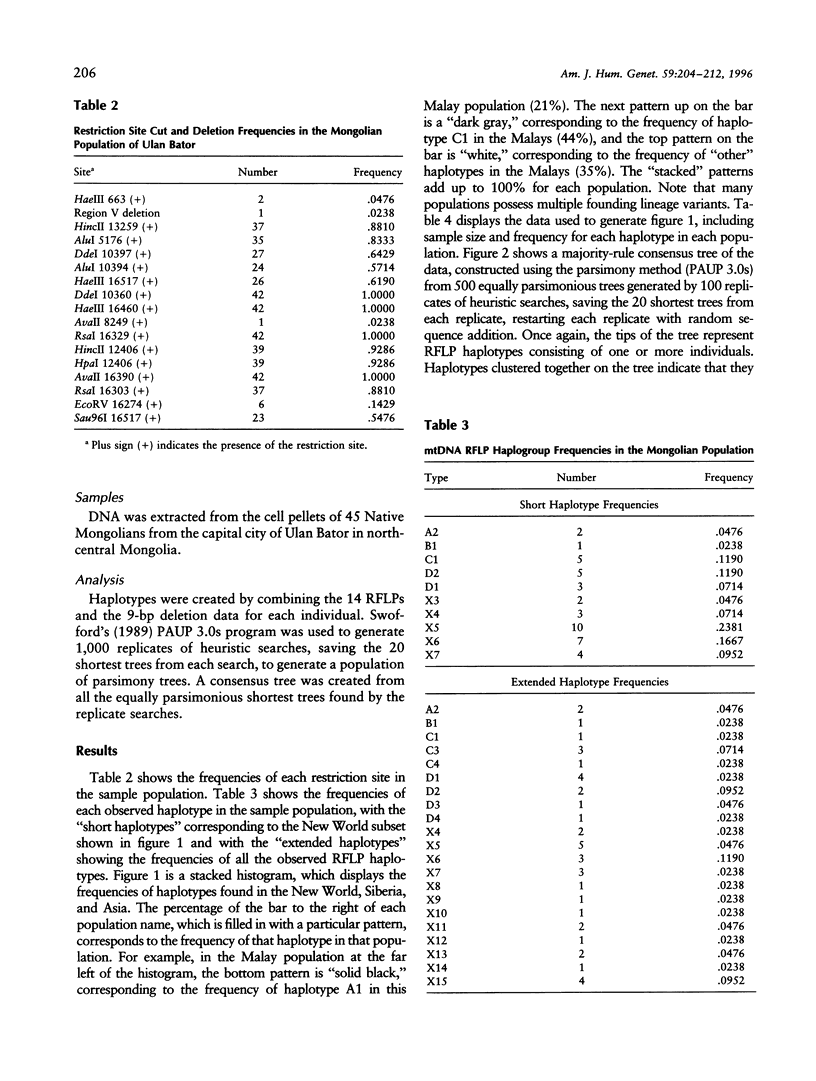
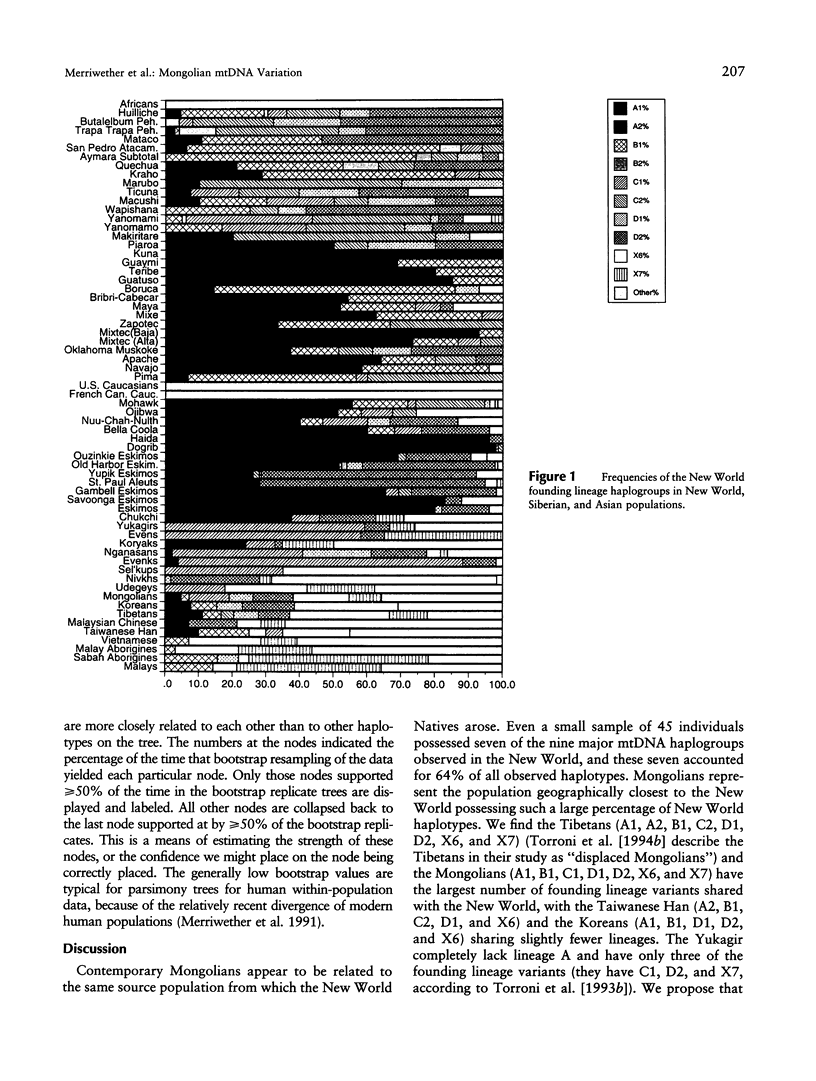
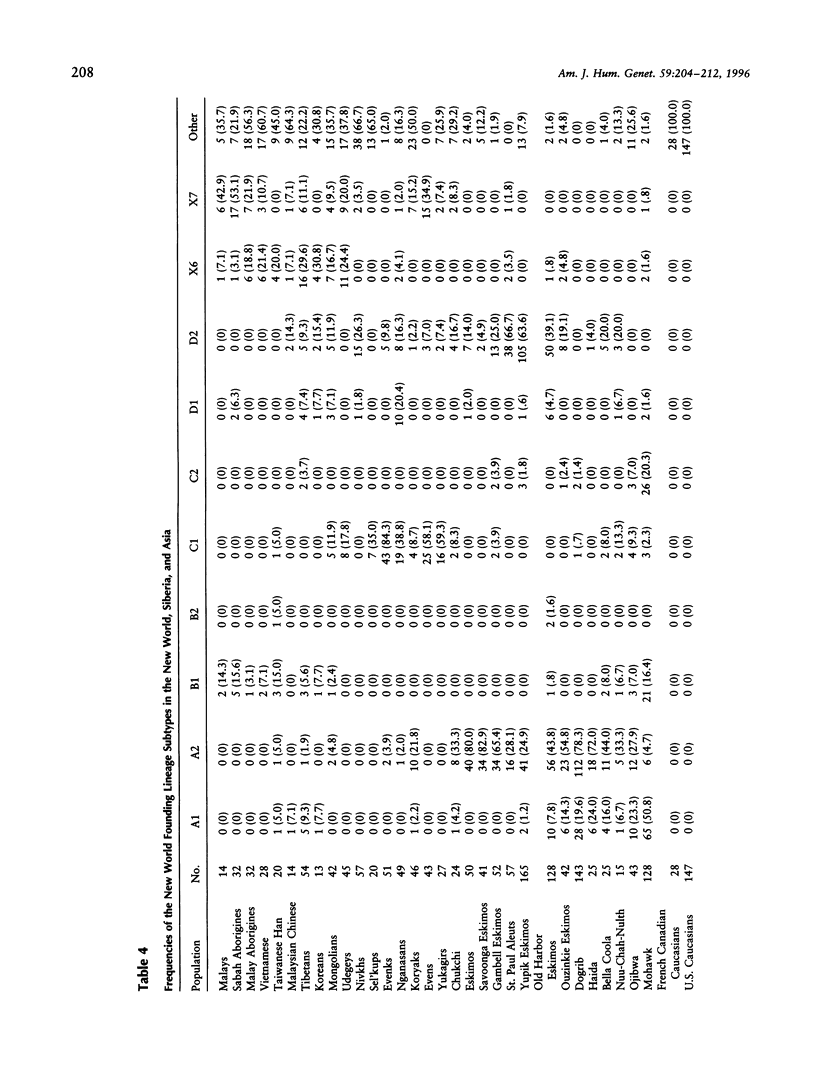
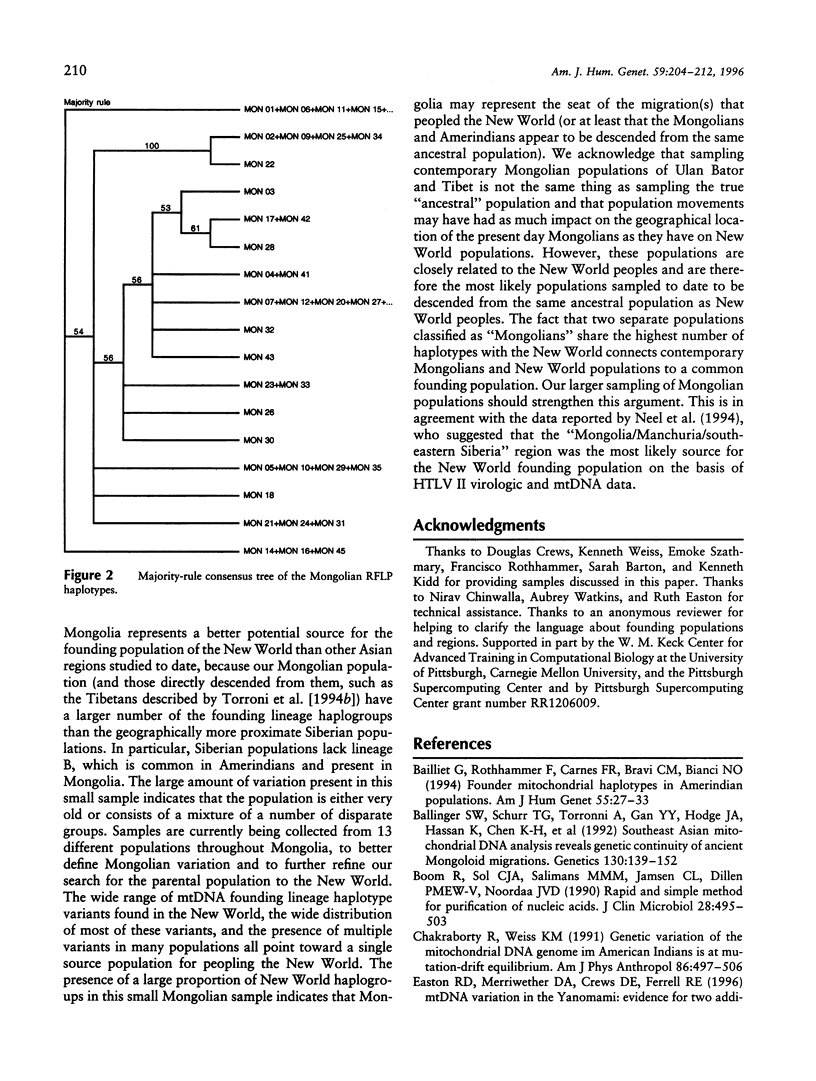
mtDNA RFLP variation was analyzed in 42 Mongolians from Ulan Bator. All four founding lineage types (A [4.76%], B [2.38%], C [11.9%], and D [19.04%]) identified by Torroni and colleagues were detected. Seven of the nine founding lineage types proposed by Bailliet and colleagues and Merriwether and Ferrell were detected (A2 [4.76%], B [2.38%], C1 [11.9%], D1 [7.14%], D2 [11.9%], X6 [16.7%], and X7 [9.5%]). Sixty-four percent of these 42 individuals had "Amerindian founding lineage" haplotypes. A survey of 24 restriction sites yielded 16 polymorphic sites and 21 different haplotypes. The presence of all four of the founding lineages identified by the Torroni group (and seven of Merriwether and Ferrell's nine founding lineages), combined with Mongolia's location with respect to the Bering Strait, indicates that Mongolia is a potential location for the origin of the founders of the New World. Since lineage B, which is widely distributed in the New World, is absent in Siberia, we conclude that Mongolia or a geographic location common to both contemporary Mongolians and American aboriginals is the more likely origin of the founders of the New World.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailliet G., Rothhammer F., Carnese F. R., Bravi C. M., Bianchi N. O. Founder mitochondrial haplotypes in Amerindian populations. Am J Hum Genet. 1994 Jul;55(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Ballinger S. W., Schurr T. G., Torroni A., Gan Y. Y., Hodge J. A., Hassan K., Chen K. H., Wallace D. C. Southeast Asian mitochondrial DNA analysis reveals genetic continuity of ancient mongoloid migrations. Genetics. 1992 Jan;130(1):139–152. doi: 10.1093/genetics/130.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R., Weiss K. M. Genetic variation of the mitochondrial DNA genome in American Indians is at mutation-drift equilibrium. Am J Phys Anthropol. 1991 Dec;86(4):497–506. doi: 10.1002/ajpa.1330860405. [DOI] [PubMed] [Google Scholar]
- Easton R. D., Merriwether D. A., Crews D. E., Ferrell R. E. mtDNA variation in the Yanomami: evidence for additional New World founding lineages. Am J Hum Genet. 1996 Jul;59(1):213–225. [PMC free article] [PubMed] [Google Scholar]
- Ginther C., Corach D., Penacino G. A., Rey J. A., Carnese F. R., Hutz M. H., Anderson A., Just J., Salzano F. M., King M. C. Genetic variation among the Mapuche Indians from the Patagonian region of Argentina: mitochondrial DNA sequence variation and allele frequencies of several nuclear genes. EXS. 1993;67:211–219. doi: 10.1007/978-3-0348-8583-6_17. [DOI] [PubMed] [Google Scholar]
- Harihara S., Hirai M., Omoto K. Mitochondrial DNA polymorphism in Japanese living in Hokkaido. Jinrui Idengaku Zasshi. 1986 Jun;31(2):73–83. doi: 10.1007/BF01871401. [DOI] [PubMed] [Google Scholar]
- Harihara S., Hirai M., Suutou Y., Shimizu K., Omoto K. Frequency of a 9-bp deletion in the mitochondrial DNA among Asian populations. Hum Biol. 1992 Apr;64(2):161–166. [PubMed] [Google Scholar]
- Harihara S., Saitou N., Hirai M., Gojobori T., Park K. S., Misawa S., Ellepola S. B., Ishida T., Omoto K. Mitochondrial DNA polymorphism among five Asian populations. Am J Hum Genet. 1988 Aug;43(2):134–143. [PMC free article] [PubMed] [Google Scholar]
- Hertzberg M., Mickleson K. N., Serjeantson S. W., Prior J. F., Trent R. J. An Asian-specific 9-bp deletion of mitochondrial DNA is frequently found in Polynesians. Am J Hum Genet. 1989 Apr;44(4):504–510. [PMC free article] [PubMed] [Google Scholar]
- Horai S., Gojobori T., Matsunaga E. Mitochondrial DNA polymorphism in Japanese. I. Analysis with restriction enzymes of six base pair recognition. Hum Genet. 1984;68(4):324–332. doi: 10.1007/BF00292594. [DOI] [PubMed] [Google Scholar]
- Horai S., Hayasaka K. Intraspecific nucleotide sequence differences in the major noncoding region of human mitochondrial DNA. Am J Hum Genet. 1990 Apr;46(4):828–842. [PMC free article] [PubMed] [Google Scholar]
- Horai S., Kondo R., Nakagawa-Hattori Y., Hayashi S., Sonoda S., Tajima K. Peopling of the Americas, founded by four major lineages of mitochondrial DNA. Mol Biol Evol. 1993 Jan;10(1):23–47. doi: 10.1093/oxfordjournals.molbev.a039987. [DOI] [PubMed] [Google Scholar]
- Horai S., Matsunaga E. Mitochondrial DNA polymorphism in Japanese. II. Analysis with restriction enzymes of four or five base pair recognition. Hum Genet. 1986 Feb;72(2):105–117. doi: 10.1007/BF00283927. [DOI] [PubMed] [Google Scholar]
- Lorenz J. G., Smith D. G. Distribution of the 9-bp mitochondrial DNA region V deletion among North American Indians. Hum Biol. 1994 Oct;66(5):777–788. [PubMed] [Google Scholar]
- Merriwether D. A., Clark A. G., Ballinger S. W., Schurr T. G., Soodyall H., Jenkins T., Sherry S. T., Wallace D. C. The structure of human mitochondrial DNA variation. J Mol Evol. 1991 Dec;33(6):543–555. doi: 10.1007/BF02102807. [DOI] [PubMed] [Google Scholar]
- Neel J. V., Biggar R. J., Sukernik R. I. Virologic and genetic studies relate Amerind origins to the indigenous people of the Mongolia/Manchuria/southeastern Siberia region. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10737–10741. doi: 10.1073/pnas.91.22.10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd A. J., Takezaki N., Sherry S. T., McGarvey S. T., Sofro A. S., Stoneking M. Evolutionary history of the COII/tRNALys intergenic 9 base pair deletion in human mitochondrial DNAs from the Pacific. Mol Biol Evol. 1995 Jul;12(4):604–615. doi: 10.1093/oxfordjournals.molbev.a040240. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schurr T. G., Ballinger S. W., Gan Y. Y., Hodge J. A., Merriwether D. A., Lawrence D. N., Knowler W. C., Weiss K. M., Wallace D. C. Amerindian mitochondrial DNAs have rare Asian mutations at high frequencies, suggesting they derived from four primary maternal lineages. Am J Hum Genet. 1990 Mar;46(3):613–623. [PMC free article] [PubMed] [Google Scholar]
- Shields G. F., Hecker K., Voevoda M. I., Reed J. K. Absence of the Asian-specific region V mitochondrial marker in Native Beringians. Am J Hum Genet. 1992 Apr;50(4):758–765. [PMC free article] [PubMed] [Google Scholar]
- Shields G. F., Schmiechen A. M., Frazier B. L., Redd A., Voevoda M. I., Reed J. K., Ward R. H. mtDNA sequences suggest a recent evolutionary divergence for Beringian and northern North American populations. Am J Hum Genet. 1993 Sep;53(3):549–562. [PMC free article] [PubMed] [Google Scholar]
- Stoneking M., Jorde L. B., Bhatia K., Wilson A. C. Geographic variation in human mitochondrial DNA from Papua New Guinea. Genetics. 1990 Mar;124(3):717–733. doi: 10.1093/genetics/124.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Chen Y. S., Semino O., Santachiara-Beneceretti A. S., Scott C. R., Lott M. T., Winter M., Wallace D. C. mtDNA and Y-chromosome polymorphisms in four Native American populations from southern Mexico. Am J Hum Genet. 1994 Feb;54(2):303–318. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Neel J. V., Barrantes R., Schurr T. G., Wallace D. C. Mitochondrial DNA "clock" for the Amerinds and its implications for timing their entry into North America. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1158–1162. doi: 10.1073/pnas.91.3.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Cabell M. F., Brown M. D., Neel J. V., Larsen M., Smith D. G., Vullo C. M., Wallace D. C. Asian affinities and continental radiation of the four founding Native American mtDNAs. Am J Hum Genet. 1993 Sep;53(3):563–590. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Yang C. C., Szathmary E. J., Williams R. C., Schanfield M. S., Troup G. A., Knowler W. C., Lawrence D. N., Weiss K. M. Native American mitochondrial DNA analysis indicates that the Amerind and the Nadene populations were founded by two independent migrations. Genetics. 1992 Jan;130(1):153–162. doi: 10.1093/genetics/130.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Sukernik R. I., Schurr T. G., Starikorskaya Y. B., Cabell M. F., Crawford M. H., Comuzzie A. G., Wallace D. C. mtDNA variation of aboriginal Siberians reveals distinct genetic affinities with Native Americans. Am J Hum Genet. 1993 Sep;53(3):591–608. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Wallace D. C. MtDNA haplogroups in Native Americans. Am J Hum Genet. 1995 May;56(5):1234–1238. [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Garrison K., Knowler W. C. Dramatic founder effects in Amerindian mitochondrial DNAs. Am J Phys Anthropol. 1985 Oct;68(2):149–155. doi: 10.1002/ajpa.1330680202. [DOI] [PubMed] [Google Scholar]
- Ward R. H., Frazier B. L., Dew-Jager K., Päbo S. Extensive mitochondrial diversity within a single Amerindian tribe. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8720–8724. doi: 10.1073/pnas.88.19.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. H., Redd A., Valencia D., Frazier B., Päbo S. Genetic and linguistic differentiation in the Americas. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10663–10667. doi: 10.1073/pnas.90.22.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]


