Abstract
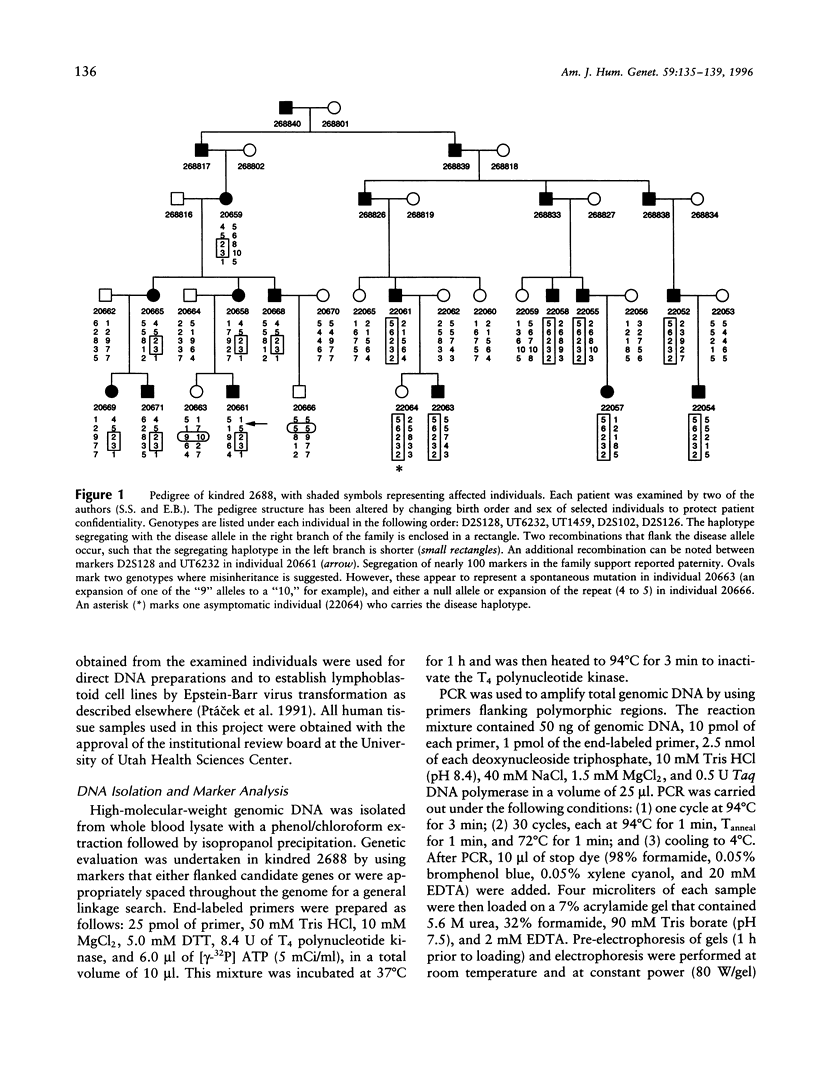
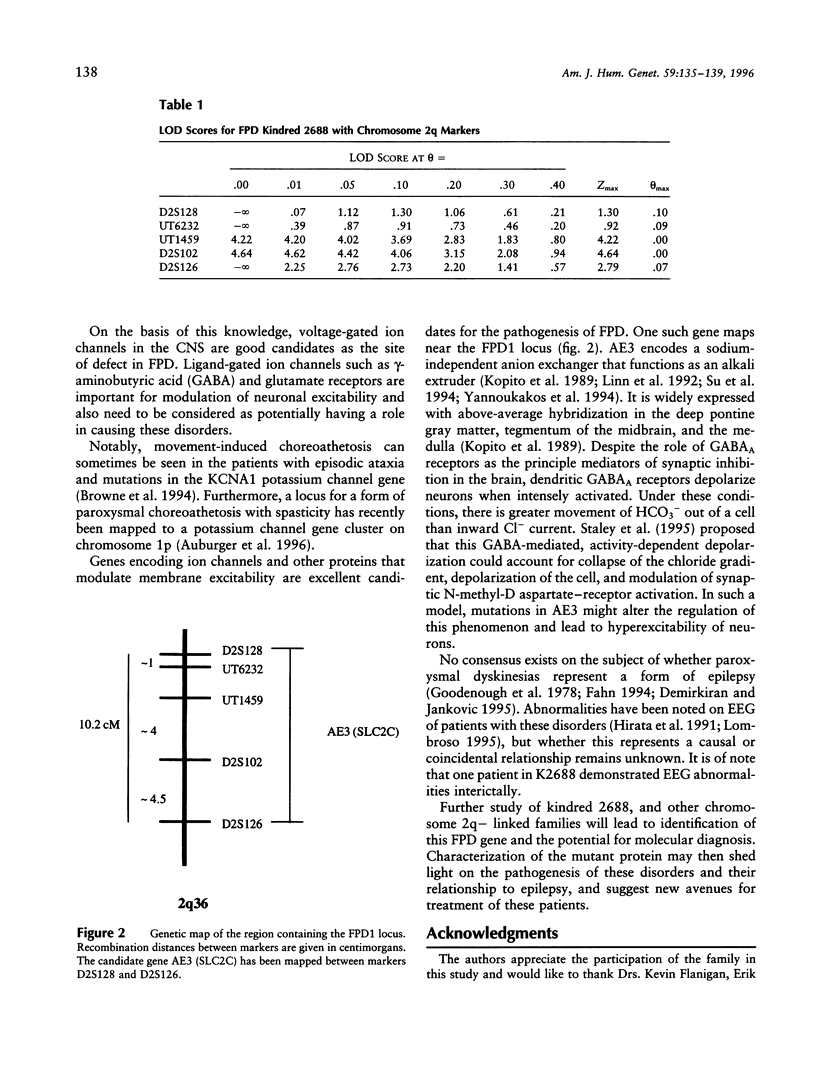
Dyskinesias are hyperkinetic and involuntary movements that may result from any of a number of different genetic, infectious, and drug-induced causes. Some of the hereditary dyskinetic syndromes are characterized by paroxysmal onset of the abnormal movements. The classification of the familial paroxysmal dyskinesias (FPD) recognizes several distinct, although overlapping, phenotypes. Different forms of the disorder include attacks that are (1) induced by sudden movement (kinesiogenic); (2) spontaneous (non-kinesiogenic); and (3) induced by prolonged periods of exertion. Linkage analysis was pursued in a family segregating an autosomal dominant allele for non-kinesiogenic FPD. The disease allele was mapped to a locus on chromosome 2q31-36 (LOD score 4.64, theta = 0). Identification of distinct genetic loci for the paroxysmal dyskinesias will lead to a new genetic classification and to better understanding of these disorders.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auburger G., Ratzlaff T., Lunkes A., Nelles H. W., Leube B., Binkofski F., Kugel H., Heindel W., Seitz R., Benecke R. A gene for autosomal dominant paroxysmal choreoathetosis/spasticity (CSE) maps to the vicinity of a potassium channel gene cluster on chromosome 1p, probably within 2 cM between D1S443 and D1S197. Genomics. 1996 Jan 1;31(1):90–94. doi: 10.1006/geno.1996.0013. [DOI] [PubMed] [Google Scholar]
- Browne D. L., Gancher S. T., Nutt J. G., Brunt E. R., Smith E. A., Kramer P., Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994 Oct;8(2):136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- Curran M. E., Splawski I., Timothy K. W., Vincent G. M., Green E. D., Keating M. T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995 Mar 10;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- Demirkiran M., Jankovic J. Paroxysmal dyskinesias: clinical features and classification. Ann Neurol. 1995 Oct;38(4):571–579. doi: 10.1002/ana.410380405. [DOI] [PubMed] [Google Scholar]
- Goodenough D. J., Fariello R. G., Annis B. L., Chun R. W. Familial and acquired paroxysmal dyskinesias. A proposed classification with delineation of clinical features. Arch Neurol. 1978 Dec;35(12):827–831. doi: 10.1001/archneur.1978.00500360051010. [DOI] [PubMed] [Google Scholar]
- Hirata K., Katayama S., Saito T., Ichihashi K., Mukai T., Katayama M., Otaka T. Paroxysmal kinesigenic choreoathetosis with abnormal electroencephalogram during attacks. Epilepsia. 1991 Jul-Aug;32(4):492–494. doi: 10.1111/j.1528-1157.1991.tb04682.x. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K., Lehmann-Horn F., Elbaz A., Heine R., Gregg R. G., Hogan K., Powers P. A., Lapie P., Vale-Santos J. E., Weissenbach J. A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet. 1994 Aug;3(8):1415–1419. doi: 10.1093/hmg/3.8.1415. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Paroxysmal kinesigenic choreoathetosis. An entity within the paroxysmal choreoathetosis syndrome. Description of 10 cases, including 1 autopsied. Neurology. 1967 Jul;17(7):680–690. doi: 10.1212/wnl.17.7.680. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Lee B. S., Simmons D. M., Lindsey A. E., Morgans C. W., Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989 Dec 1;59(5):927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet. 1985 May;37(3):482–498. [PMC free article] [PubMed] [Google Scholar]
- Linn S. C., Kudrycki K. E., Shull G. E. The predicted translation product of a cardiac AE3 mRNA contains an N terminus distinct from that of the brain AE3 Cl-/HCO3- exchanger. Cloning of a cardiac AE3 cDNA, organization of the AE3 gene, and identification of an alternative transcription initiation site. J Biol Chem. 1992 Apr 15;267(11):7927–7935. [PubMed] [Google Scholar]
- Lombroso C. T. Paroxysmal choreoathetosis: an epileptic or non-epileptic disorder? Ital J Neurol Sci. 1995 Jun;16(5):271–277. doi: 10.1007/BF02249102. [DOI] [PubMed] [Google Scholar]
- McClatchey A. I., Van den Bergh P., Pericak-Vance M. A., Raskind W., Verellen C., McKenna-Yasek D., Rao K., Haines J. L., Bird T., Brown R. H., Jr Temperature-sensitive mutations in the III-IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell. 1992 Feb 21;68(4):769–774. doi: 10.1016/0092-8674(92)90151-2. [DOI] [PubMed] [Google Scholar]
- Ptacek L. J., Gouw L., Kwieciński H., McManis P., Mendell J. R., Barohn R. J., George A. L., Jr, Barchi R. L., Robertson M., Leppert M. F. Sodium channel mutations in paramyotonia congenita and hyperkalemic periodic paralysis. Ann Neurol. 1993 Mar;33(3):300–307. doi: 10.1002/ana.410330312. [DOI] [PubMed] [Google Scholar]
- Ptácek L. J., George A. L., Jr, Barchi R. L., Griggs R. C., Riggs J. E., Robertson M., Leppert M. F. Mutations in an S4 segment of the adult skeletal muscle sodium channel cause paramyotonia congenita. Neuron. 1992 May;8(5):891–897. doi: 10.1016/0896-6273(92)90203-p. [DOI] [PubMed] [Google Scholar]
- Ptácek L. J., George A. L., Jr, Griggs R. C., Tawil R., Kallen R. G., Barchi R. L., Robertson M., Leppert M. F. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell. 1991 Nov 29;67(5):1021–1027. doi: 10.1016/0092-8674(91)90374-8. [DOI] [PubMed] [Google Scholar]
- Ptácek L. J., Tawil R., Griggs R. C., Engel A. G., Layzer R. B., Kwieciński H., McManis P. G., Santiago L., Moore M., Fouad G. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell. 1994 Jun 17;77(6):863–868. doi: 10.1016/0092-8674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Rojas C. V., Wang J. Z., Schwartz L. S., Hoffman E. P., Powell B. R., Brown R. H., Jr A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature. 1991 Dec 5;354(6352):387–389. doi: 10.1038/354387a0. [DOI] [PubMed] [Google Scholar]
- Shiang R., Ryan S. G., Zhu Y. Z., Hahn A. F., O'Connell P., Wasmuth J. J. Mutations in the alpha 1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat Genet. 1993 Dec;5(4):351–358. doi: 10.1038/ng1293-351. [DOI] [PubMed] [Google Scholar]
- Staley K. J., Soldo B. L., Proctor W. R. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995 Aug 18;269(5226):977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Su Y. R., Klanke C. A., Houseal T. W., Linn S. C., Burk S. E., Varvil T. S., Otterud B. E., Shull G. E., Leppert M. F., Menon A. G. Molecular cloning and physical and genetic mapping of the human anion exchanger isoform 3 (SLC2C) gene to chromosome 2q36. Genomics. 1994 Aug;22(3):605–609. doi: 10.1006/geno.1994.1433. [DOI] [PubMed] [Google Scholar]
- Wang Q., Curran M. E., Splawski I., Burn T. C., Millholland J. M., VanRaay T. J., Shen J., Timothy K. W., Vincent G. M., de Jager T. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996 Jan;12(1):17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Wang Q., Shen J., Splawski I., Atkinson D., Li Z., Robinson J. L., Moss A. J., Towbin J. A., Keating M. T. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995 Mar 10;80(5):805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- Yannoukakos D., Stuart-Tilley A., Fernandez H. A., Fey P., Duyk G., Alper S. L. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ Res. 1994 Oct;75(4):603–614. doi: 10.1161/01.res.75.4.603. [DOI] [PubMed] [Google Scholar]


