Abstract
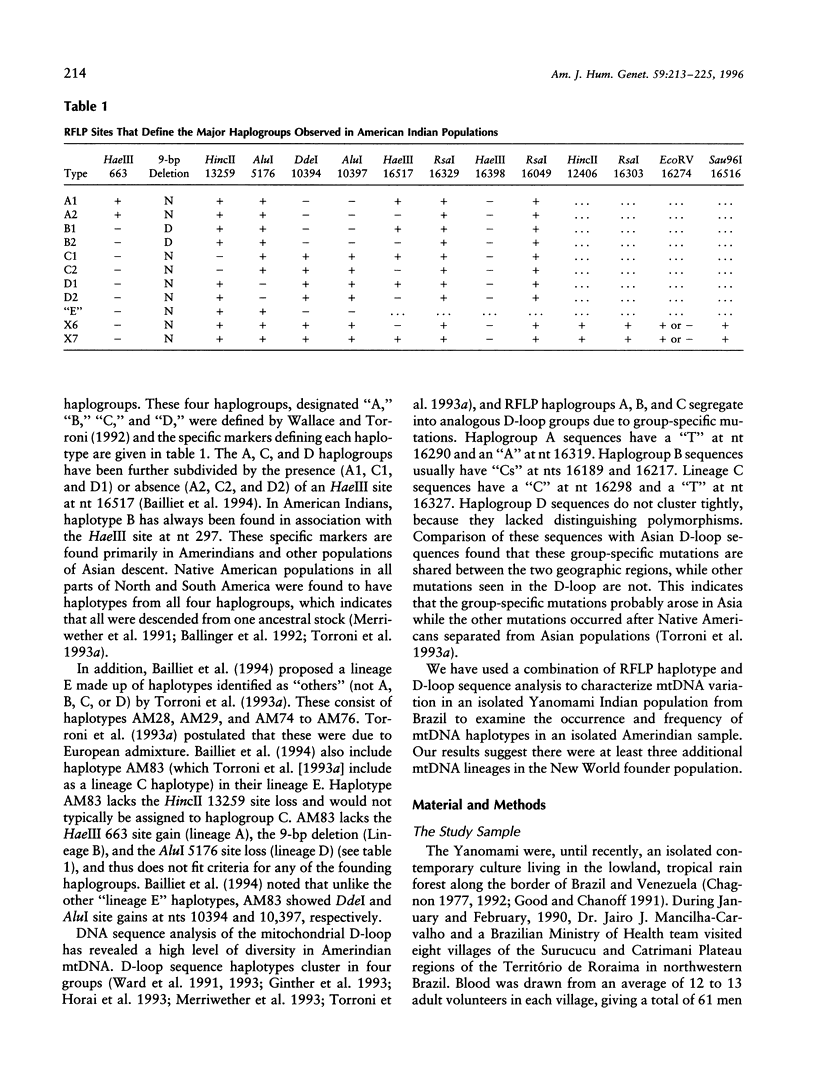
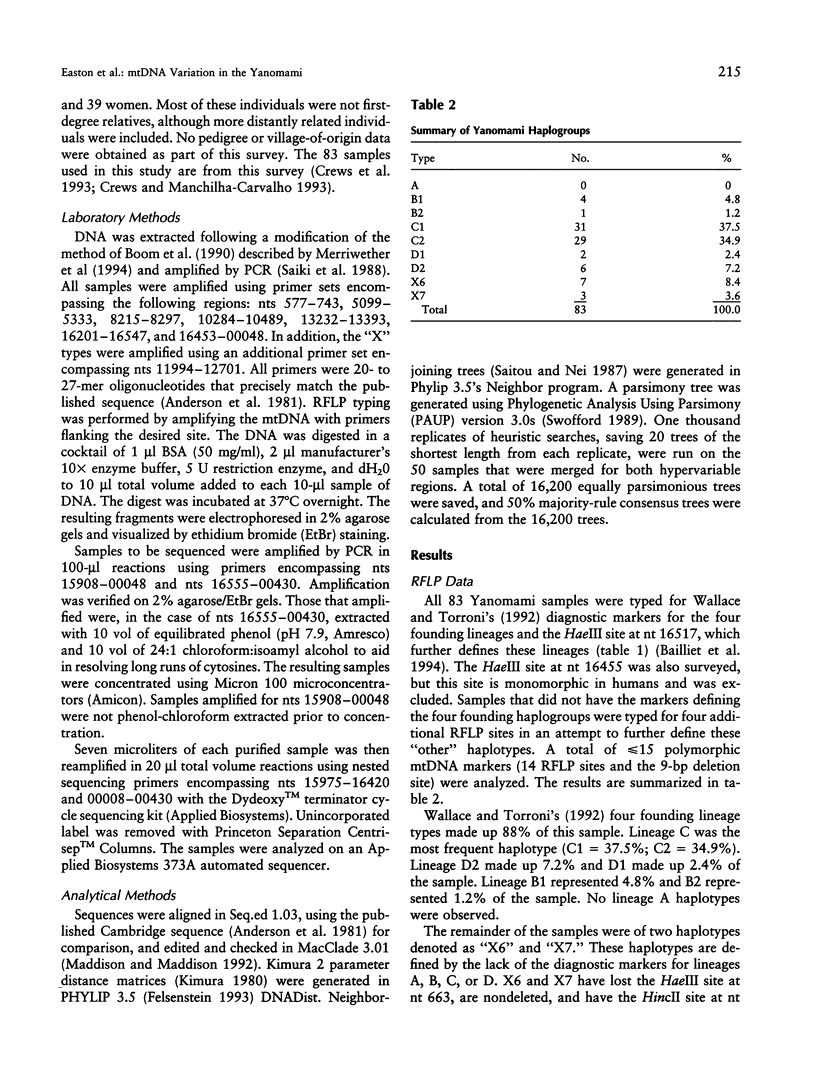
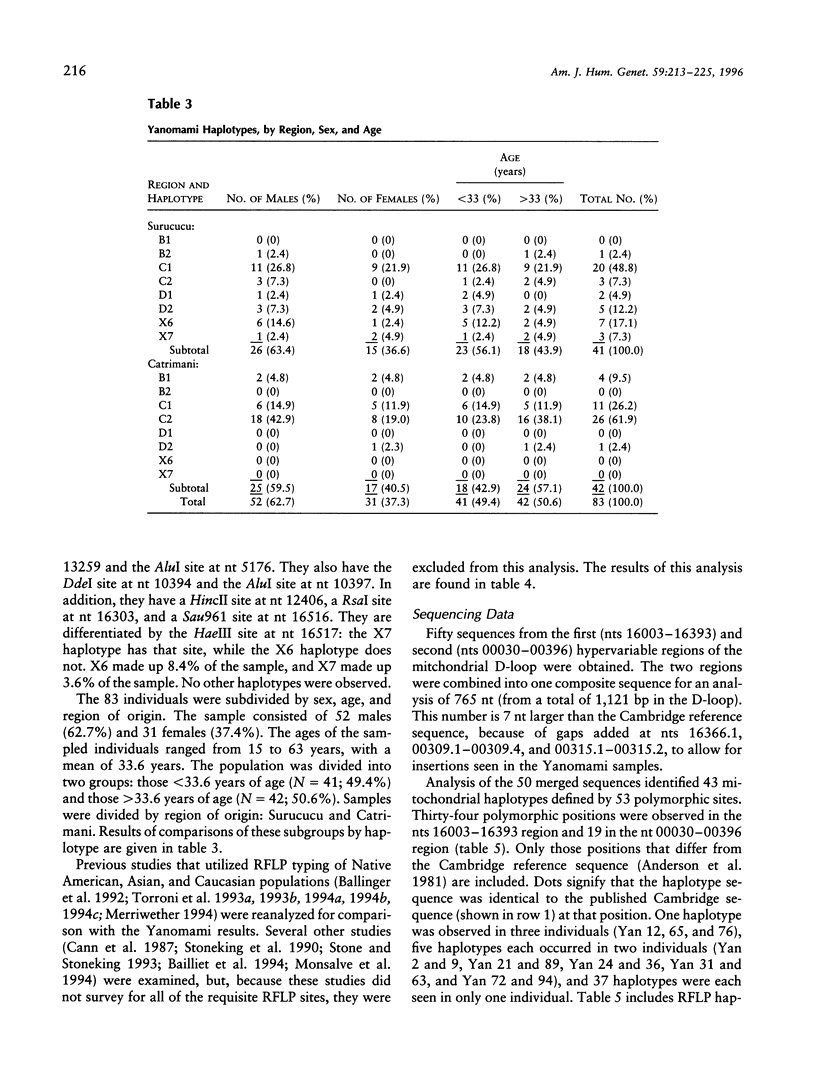
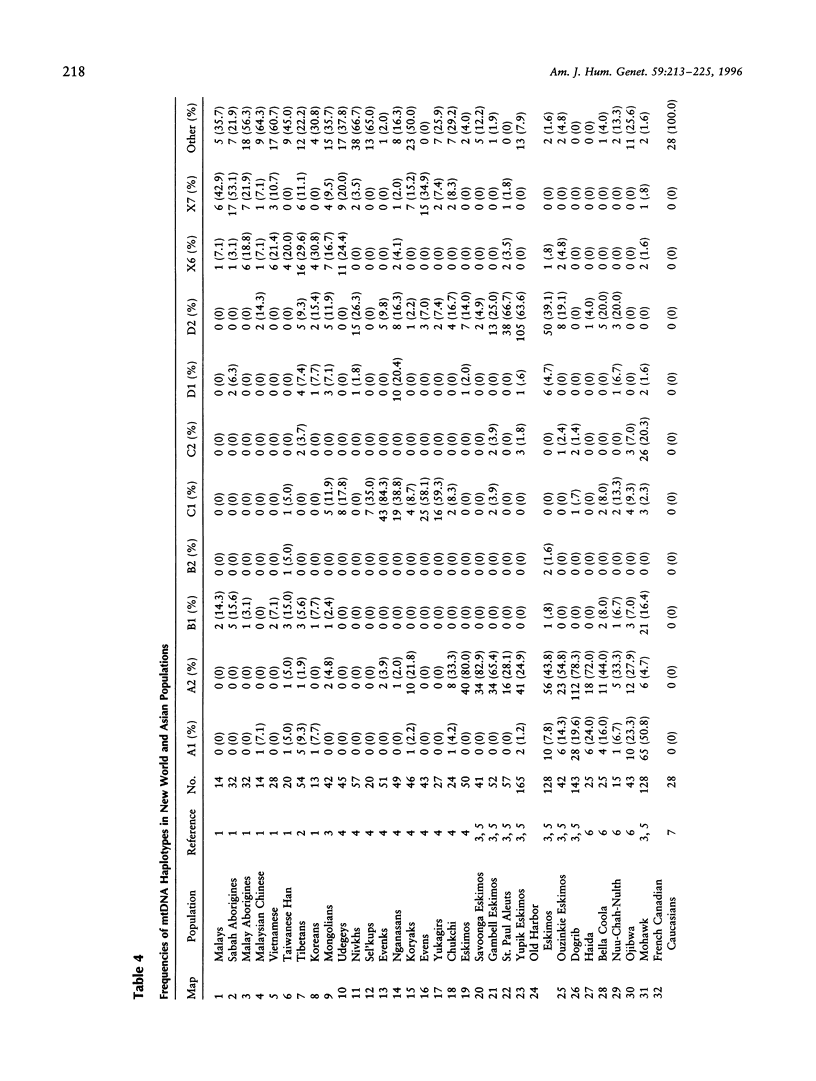
Native Americans have been classified into four founding haplogroups with as many as seven founding lineages based on mtDNA RFLPs and DNA sequence data. mtDNA analysis was completed for 83 Yanomami from eight villages in the Surucucu and Catrimani Plateau regions of Roraima in northwestern Brazil. Samples were typed for 15 polymorphic mtDNA sites (14 RFLP sites and 1 deletion site), and a subset was sequenced for both hypervariable regions of the mitochondrial D-loop. Substantial mitochondrial diversity was detected among the Yanomami, five of seven accepted founding haplotypes and three others were observed. Of the 83 samples, 4 (4.8%) were lineage B1, 1 (1.2%) was lineage B2, 31 (37.4%) were lineage C1, 29 (34.9%) were lineage C2, 2 (2.4%) were lineage D1, 6 (7.2%) were lineage D2, 7 (8.4%) were a haplotype we designated "X6," and 3 (3.6%) were a haplotype we designated "X7." Sequence analysis found 43 haplotypes in 50 samples. B2, X6, and X7 are previously unrecognized mitochondrial founding lineage types of Native Americans. The widespread distribution of these haplotypes in the New World and Asia provides support for declaring these lineages to be New World founding types.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailliet G., Rothhammer F., Carnese F. R., Bravi C. M., Bianchi N. O. Founder mitochondrial haplotypes in Amerindian populations. Am J Hum Genet. 1994 Jul;55(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Ballinger S. W., Schurr T. G., Torroni A., Gan Y. Y., Hodge J. A., Hassan K., Chen K. H., Wallace D. C. Southeast Asian mitochondrial DNA analysis reveals genetic continuity of ancient mongoloid migrations. Genetics. 1992 Jan;130(1):139–152. doi: 10.1093/genetics/130.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Cann R. L. mtDNA and Native Americans: a Southern perspective. Am J Hum Genet. 1994 Jul;55(1):7–11. [PMC free article] [PubMed] [Google Scholar]
- Chen Y. S., Torroni A., Excoffier L., Santachiara-Benerecetti A. S., Wallace D. C. Analysis of mtDNA variation in African populations reveals the most ancient of all human continent-specific haplogroups. Am J Hum Genet. 1995 Jul;57(1):133–149. [PMC free article] [PubMed] [Google Scholar]
- Crews D. E., Kamboh M. I., Mancilha-Carvalho J. J., Kottke B. Population genetics of apolipoprotein A-4, E, and H polymorphisms in Yanomami Indians of northwestern Brazil: associations with lipids, lipoproteins, and carbohydrate metabolism. Hum Biol. 1993 Apr;65(2):211–224. [PubMed] [Google Scholar]
- Crews D. E., Mancilha-Carvalho J. J. Correlates of blood pressure in Yanomami Indians of northwestern Brazil. Ethn Dis. 1993 Fall;3(4):362–371. [PubMed] [Google Scholar]
- Doran G. H., Dickel D. N., Ballinger W. E., Jr, Agee O. F., Laipis P. J., Hauswirth W. W. Anatomical, cellular and molecular analysis of 8,000-yr-old human brain tissue from the Windover archaeological site. 1986 Oct 30-Nov 5Nature. 323(6091):803–806. doi: 10.1038/323803a0. [DOI] [PubMed] [Google Scholar]
- Ginther C., Corach D., Penacino G. A., Rey J. A., Carnese F. R., Hutz M. H., Anderson A., Just J., Salzano F. M., King M. C. Genetic variation among the Mapuche Indians from the Patagonian region of Argentina: mitochondrial DNA sequence variation and allele frequencies of several nuclear genes. EXS. 1993;67:211–219. doi: 10.1007/978-3-0348-8583-6_17. [DOI] [PubMed] [Google Scholar]
- Horai S., Kondo R., Nakagawa-Hattori Y., Hayashi S., Sonoda S., Tajima K. Peopling of the Americas, founded by four major lineages of mitochondrial DNA. Mol Biol Evol. 1993 Jan;10(1):23–47. doi: 10.1093/oxfordjournals.molbev.a039987. [DOI] [PubMed] [Google Scholar]
- Johnson M. J., Wallace D. C., Ferris S. D., Rattazzi M. C., Cavalli-Sforza L. L. Radiation of human mitochondria DNA types analyzed by restriction endonuclease cleavage patterns. J Mol Evol. 1983;19(3-4):255–271. doi: 10.1007/BF02099973. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Marshall E. Clovis Counterrevolution: The debate on who came first to the Americas, and where they lived, seems headed for a new round as the skeptics strike at "pre-Clovis" discoveries. Science. 1990 Aug 17;249(4970):738–741. doi: 10.1126/science.249.4970.738. [DOI] [PubMed] [Google Scholar]
- Merriwether D. A., Clark A. G., Ballinger S. W., Schurr T. G., Soodyall H., Jenkins T., Sherry S. T., Wallace D. C. The structure of human mitochondrial DNA variation. J Mol Evol. 1991 Dec;33(6):543–555. doi: 10.1007/BF02102807. [DOI] [PubMed] [Google Scholar]
- Merriwether D. A., Hall W. W., Vahlne A., Ferrell R. E. mtDNA variation indicates Mongolia may have been the source for the founding population for the New World. Am J Hum Genet. 1996 Jul;59(1):204–212. [PMC free article] [PubMed] [Google Scholar]
- Monsalve M. V., Groot de Restrepo H., Espinel A., Correal G., Devine D. V. Evidence of mitochondrial DNA diversity in South American aboriginals. Ann Hum Genet. 1994 Jul;58(Pt 3):265–273. doi: 10.1111/j.1469-1809.1994.tb01890.x. [DOI] [PubMed] [Google Scholar]
- Redd A. J., Takezaki N., Sherry S. T., McGarvey S. T., Sofro A. S., Stoneking M. Evolutionary history of the COII/tRNALys intergenic 9 base pair deletion in human mitochondrial DNAs from the Pacific. Mol Biol Evol. 1995 Jul;12(4):604–615. doi: 10.1093/oxfordjournals.molbev.a040240. [DOI] [PubMed] [Google Scholar]
- Rothhammer F., Bianchi N. O. Origin and distribution of B mtDNA lineage in South America. Am J Hum Genet. 1995 May;56(5):1247–1248. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schurr T. G., Ballinger S. W., Gan Y. Y., Hodge J. A., Merriwether D. A., Lawrence D. N., Knowler W. C., Weiss K. M., Wallace D. C. Amerindian mitochondrial DNAs have rare Asian mutations at high frequencies, suggesting they derived from four primary maternal lineages. Am J Hum Genet. 1990 Mar;46(3):613–623. [PMC free article] [PubMed] [Google Scholar]
- Shields G. F., Schmiechen A. M., Frazier B. L., Redd A., Voevoda M. I., Reed J. K., Ward R. H. mtDNA sequences suggest a recent evolutionary divergence for Beringian and northern North American populations. Am J Hum Genet. 1993 Sep;53(3):549–562. [PMC free article] [PubMed] [Google Scholar]
- Stone A. C., Stoneking M. Ancient DNA from a pre-Columbian Amerindian population. Am J Phys Anthropol. 1993 Dec;92(4):463–471. doi: 10.1002/ajpa.1330920405. [DOI] [PubMed] [Google Scholar]
- Stoneking M., Jorde L. B., Bhatia K., Wilson A. C. Geographic variation in human mitochondrial DNA from Papua New Guinea. Genetics. 1990 Mar;124(3):717–733. doi: 10.1093/genetics/124.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szathmary E. J. mtDNA and the peopling of the Americas. Am J Hum Genet. 1993 Oct;53(4):793–799. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Chen Y. S., Semino O., Santachiara-Beneceretti A. S., Scott C. R., Lott M. T., Winter M., Wallace D. C. mtDNA and Y-chromosome polymorphisms in four Native American populations from southern Mexico. Am J Hum Genet. 1994 Feb;54(2):303–318. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Lott M. T., Cabell M. F., Chen Y. S., Lavergne L., Wallace D. C. mtDNA and the origin of Caucasians: identification of ancient Caucasian-specific haplogroups, one of which is prone to a recurrent somatic duplication in the D-loop region. Am J Hum Genet. 1994 Oct;55(4):760–776. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Miller J. A., Moore L. G., Zamudio S., Zhuang J., Droma T., Wallace D. C. Mitochondrial DNA analysis in Tibet: implications for the origin of the Tibetan population and its adaptation to high altitude. Am J Phys Anthropol. 1994 Feb;93(2):189–199. doi: 10.1002/ajpa.1330930204. [DOI] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Cabell M. F., Brown M. D., Neel J. V., Larsen M., Smith D. G., Vullo C. M., Wallace D. C. Asian affinities and continental radiation of the four founding Native American mtDNAs. Am J Hum Genet. 1993 Sep;53(3):563–590. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Sukernik R. I., Schurr T. G., Starikorskaya Y. B., Cabell M. F., Crawford M. H., Comuzzie A. G., Wallace D. C. mtDNA variation of aboriginal Siberians reveals distinct genetic affinities with Native Americans. Am J Hum Genet. 1993 Sep;53(3):591–608. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Wallace D. C. MtDNA haplogroups in Native Americans. Am J Hum Genet. 1995 May;56(5):1234–1238. [PMC free article] [PubMed] [Google Scholar]
- Vigilant L., Pennington R., Harpending H., Kocher T. D., Wilson A. C. Mitochondrial DNA sequences in single hairs from a southern African population. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9350–9354. doi: 10.1073/pnas.86.23.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigilant L., Stoneking M., Harpending H., Hawkes K., Wilson A. C. African populations and the evolution of human mitochondrial DNA. Science. 1991 Sep 27;253(5027):1503–1507. doi: 10.1126/science.1840702. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Garrison K., Knowler W. C. Dramatic founder effects in Amerindian mitochondrial DNAs. Am J Phys Anthropol. 1985 Oct;68(2):149–155. doi: 10.1002/ajpa.1330680202. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Torroni A. American Indian prehistory as written in the mitochondrial DNA: a review. Hum Biol. 1992 Jun;64(3):403–416. [PubMed] [Google Scholar]
- Ward R. H., Frazier B. L., Dew-Jager K., Päbo S. Extensive mitochondrial diversity within a single Amerindian tribe. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8720–8724. doi: 10.1073/pnas.88.19.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. H., Redd A., Valencia D., Frazier B., Päbo S. Genetic and linguistic differentiation in the Americas. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10663–10667. doi: 10.1073/pnas.90.22.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]


