Abstract
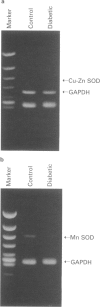
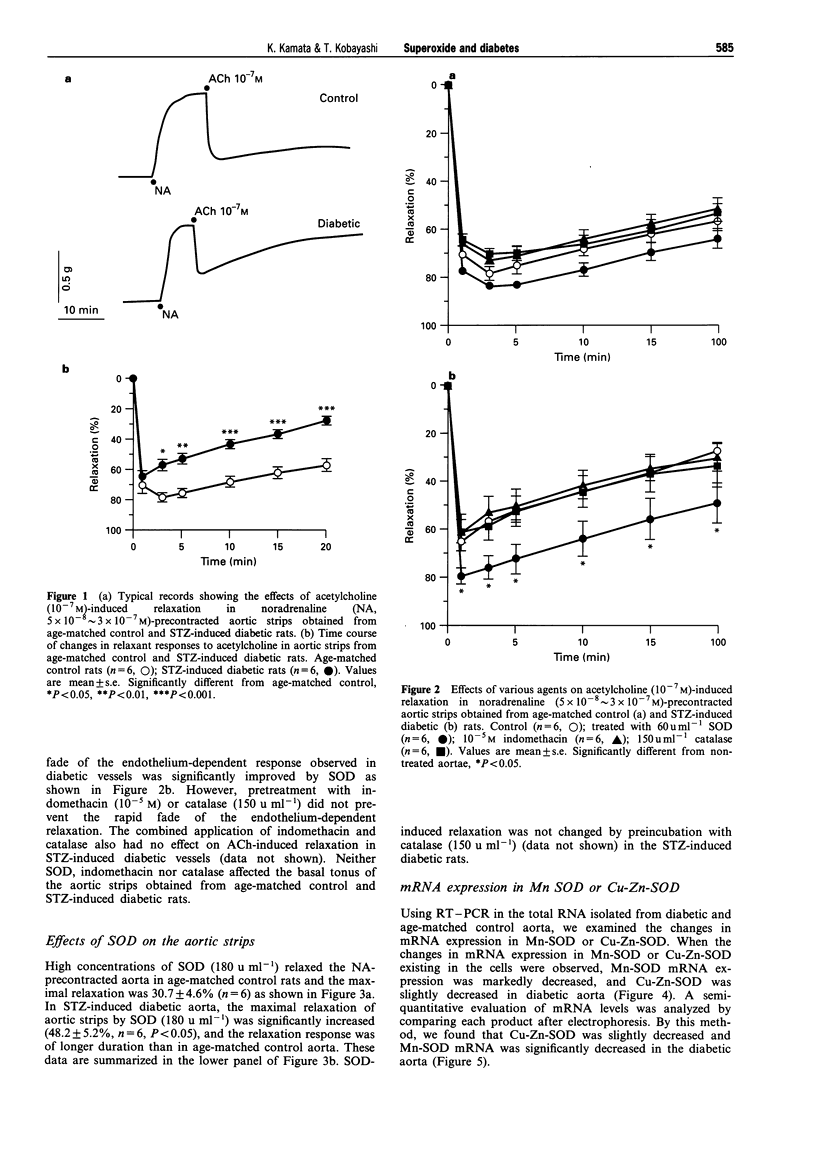
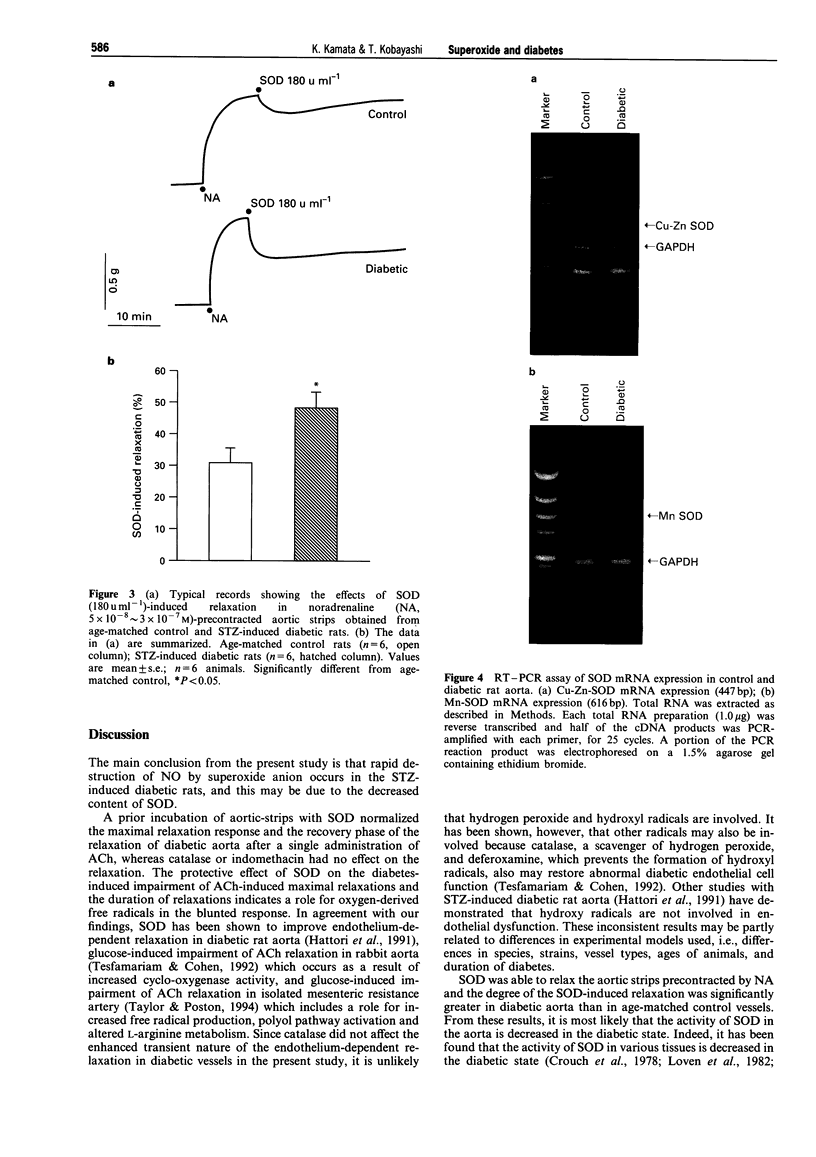
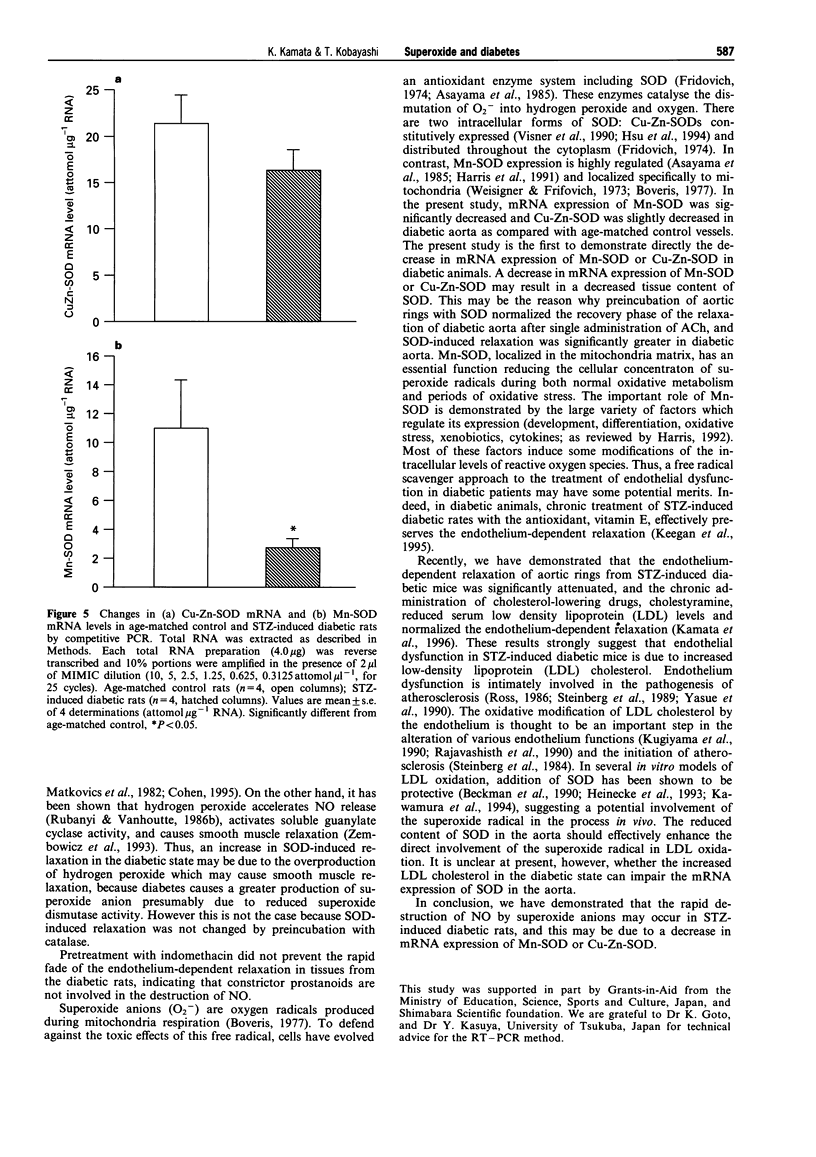
1. Experiments were designed to investigate the involvement of superoxide anions in the attenuated endothelium-dependent relaxation of the rat aorta from streptozotocin (STZ)-induced diabetic rats. 2. The endothelium-dependent relaxation responses to acetylcholine (ACh, 10(-7) M) in helical strips of the aorta precontracted with noradrenaline (NA, 5 x 10(-3) approximately 3 x 10(-7) M) were significantly decreased in STZ-induced diabetic rats. The recovery phase of the relaxation after single administration of ACh in the STZ-induced diabetic rats was more rapid than those in control vessels. 3. Preincubation of aortic strips with superoxide dismutase (SOD, 60 u ml-1) normalized the recovery phase of the relaxation of diabetic aorta after single administration of ACh, whereas catalase (150 u ml-1) or indomethacin (10(-5) M) had no effects on the relaxation. 4. SOD (180 u ml-1) caused relaxation in NA precontracted aortic strips and the degree of the SOD-induced relaxation was significantly greater in diabetic aorta as compared with age-matched control vessels. 5. When the changes in mRNA expressions of Mn-SOD or Cu-Zn-SOD were observed, Mn-SOD mRNA expression was markedly decreased, and Cu-Zn-SOD was slightly decreased in diabetic aorta. 6. These results suggest that the rapid destruction of NO by superoxide anions may occur in the STZ-induced diabetic rats, and this may be due to a decrease in mRNA expression of Mn-SOD or Cu-Zn-SOD.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asayama K., Janco R. L., Burr I. M. Selective induction of manganous superoxide dismutase in human monocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):C393–C397. doi: 10.1152/ajpcell.1985.249.5.C393. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christlieb A. R. Diabetes and hypertensive vascular disease. Mechanisms and treatment. Am J Cardiol. 1973 Sep 20;32(4):592–606. doi: 10.1016/s0002-9149(73)80051-7. [DOI] [PubMed] [Google Scholar]
- Cohen R. A. The role of nitric oxide and other endothelium-derived vasoactive substances in vascular disease. Prog Cardiovasc Dis. 1995 Sep-Oct;38(2):105–128. doi: 10.1016/s0033-0620(05)80002-7. [DOI] [PubMed] [Google Scholar]
- Crouch R., Kimsey G., Priest D. G., Sarda A., Buse M. G. Effect of streptozotocin on erythrocyte and retinal superoxide dismutase. Diabetologia. 1978 Jul;15(1):53–57. doi: 10.1007/BF01219329. [DOI] [PubMed] [Google Scholar]
- DUBOWSKI K. M. An o-toluidine method for body-fluid glucose determination. Clin Chem. 1962 May-Jun;8:215–235. [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Harris C. A., Derbin K. S., Hunte-McDonough B., Krauss M. R., Chen K. T., Smith D. M., Epstein L. B. Manganese superoxide dismutase is induced by IFN-gamma in multiple cell types. Synergistic induction by IFN-gamma and tumor necrosis factor or IL-1. J Immunol. 1991 Jul 1;147(1):149–154. [PubMed] [Google Scholar]
- Harris E. D. Regulation of antioxidant enzymes. FASEB J. 1992 Jun;6(9):2675–2683. doi: 10.1096/fasebj.6.9.1612291. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Kawasaki H., Abe K., Kanno M. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1991 Oct;261(4 Pt 2):H1086–H1094. doi: 10.1152/ajpheart.1991.261.4.H1086. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Kawamura M., Suzuki L., Chait A. Oxidation of low density lipoprotein by thiols: superoxide-dependent and -independent mechanisms. J Lipid Res. 1993 Dec;34(12):2051–2061. [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989 Jun;97(2):614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Involvement of endothelial cells in relaxation and contraction responses of the aorta to isoproterenol in naive and streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 1989 Jun;249(3):890–894. [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Mechanisms of increased responses of the aorta to alpha-adrenoceptor agonists in streptozotocin-induced diabetic rats. J Pharmacobiodyn. 1988 Oct;11(10):707–713. doi: 10.1248/bpb1978.11.707. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Heinecke J. W., Chait A. Pathophysiological concentrations of glucose promote oxidative modification of low density lipoprotein by a superoxide-dependent pathway. J Clin Invest. 1994 Aug;94(2):771–778. doi: 10.1172/JCI117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan A., Walbank H., Cotter M. A., Cameron N. E. Chronic vitamin E treatment prevents defective endothelium-dependent relaxation in diabetic rat aorta. Diabetologia. 1995 Dec;38(12):1475–1478. doi: 10.1007/BF00400609. [DOI] [PubMed] [Google Scholar]
- Kugiyama K., Kerns S. A., Morrisett J. D., Roberts R., Henry P. D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990 Mar 8;344(6262):160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- Langenstroer P., Pieper G. M. Regulation of spontaneous EDRF release in diabetic rat aorta by oxygen free radicals. Am J Physiol. 1992 Jul;263(1 Pt 2):H257–H265. doi: 10.1152/ajpheart.1992.263.1.H257. [DOI] [PubMed] [Google Scholar]
- Loven D. P., Schedl H. P., Oberley L. W., Wilson H. D., Bruch L., Niehaus C. L. Superoxide dismutase activity in the intestine of the streptozotocin-diabetic rat. Endocrinology. 1982 Sep;111(3):737–742. doi: 10.1210/endo-111-3-737. [DOI] [PubMed] [Google Scholar]
- Matkovics B., Varga S. I., Szabó L., Witas H. The effect of diabetes on the activities of the peroxide metabolism enzymes. Horm Metab Res. 1982 Feb;14(2):77–79. doi: 10.1055/s-2007-1018928. [DOI] [PubMed] [Google Scholar]
- Mian K. B., Martin W. Differential sensitivity of basal and acetylcholine-stimulated activity of nitric oxide to destruction by superoxide anion in rat aorta. Br J Pharmacol. 1995 Jul;115(6):993–1000. doi: 10.1111/j.1476-5381.1995.tb15909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Pannetier C., Delassus S., Darche S., Saucier C., Kourilsky P. Quantitative titration of nucleic acids by enzymatic amplification reactions run to saturation. Nucleic Acids Res. 1993 Feb 11;21(3):577–583. doi: 10.1093/nar/21.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper G. M., Gross G. J. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1988 Oct;255(4 Pt 2):H825–H833. doi: 10.1152/ajpheart.1988.255.4.H825. [DOI] [PubMed] [Google Scholar]
- Pieper G. M., Mei D. A., Langenstroer P., O'Rourke S. T. Bioassay of endothelium-derived relaxing factor in diabetic rat aorta. Am J Physiol. 1992 Sep;263(3 Pt 2):H676–H680. doi: 10.1152/ajpheart.1992.263.3.H676. [DOI] [PubMed] [Google Scholar]
- Porcher C., Malinge M. C., Picat C., Grandchamp B. A simplified method for determination of specific DNA or RNA copy number using quantitative PCR and an automatic DNA sequencer. Biotechniques. 1992 Jul;13(1):106–114. [PubMed] [Google Scholar]
- Poston L., Taylor P. D. Glaxo/MRS Young Investigator Prize. Endothelium-mediated vascular function in insulin-dependent diabetes mellitus. Clin Sci (Lond) 1995 Mar;88(3):245–255. doi: 10.1042/cs0880245. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986 May;250(5 Pt 2):H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. D., Poston L. The effect of hyperglycaemia on function of rat isolated mesenteric resistance artery. Br J Pharmacol. 1994 Nov;113(3):801–808. doi: 10.1111/j.1476-5381.1994.tb17064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Cohen R. A. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992 Aug;263(2 Pt 2):H321–H326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- Tomlinson K. C., Gardiner S. M., Hebden R. A., Bennett T. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol Rev. 1992 Mar;44(1):103–150. [PubMed] [Google Scholar]
- Visner G. A., Dougall W. C., Wilson J. M., Burr I. A., Nick H. S. Regulation of manganese superoxide dismutase by lipopolysaccharide, interleukin-1, and tumor necrosis factor. Role in the acute inflammatory response. J Biol Chem. 1990 Feb 15;265(5):2856–2864. [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Yasue H., Matsuyama K., Matsuyama K., Okumura K., Morikami Y., Ogawa H. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment. Possible role of early coronary atherosclerosis. Circulation. 1990 Feb;81(2):482–490. doi: 10.1161/01.cir.81.2.482. [DOI] [PubMed] [Google Scholar]
- Zembowicz A., Hatchett R. J., Jakubowski A. M., Gryglewski R. J. Involvement of nitric oxide in the endothelium-dependent relaxation induced by hydrogen peroxide in the rabbit aorta. Br J Pharmacol. 1993 Sep;110(1):151–158. doi: 10.1111/j.1476-5381.1993.tb13785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]