Abstract
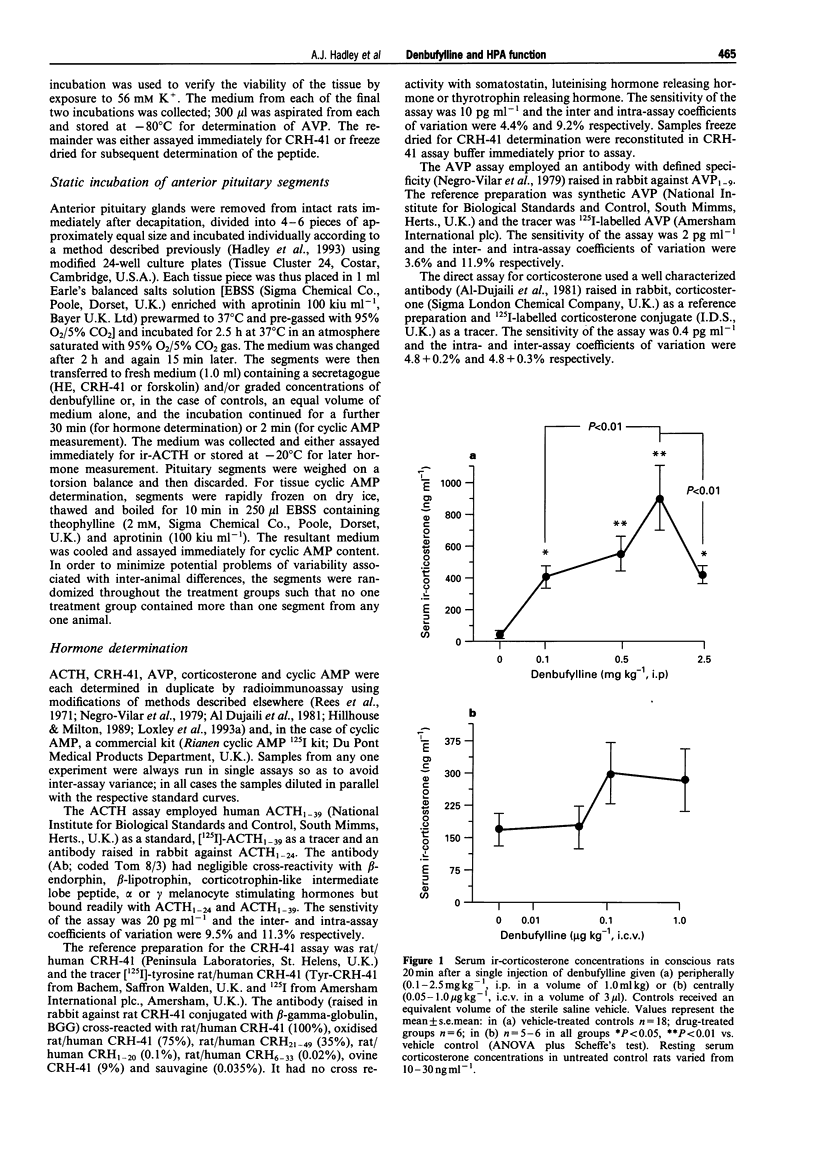
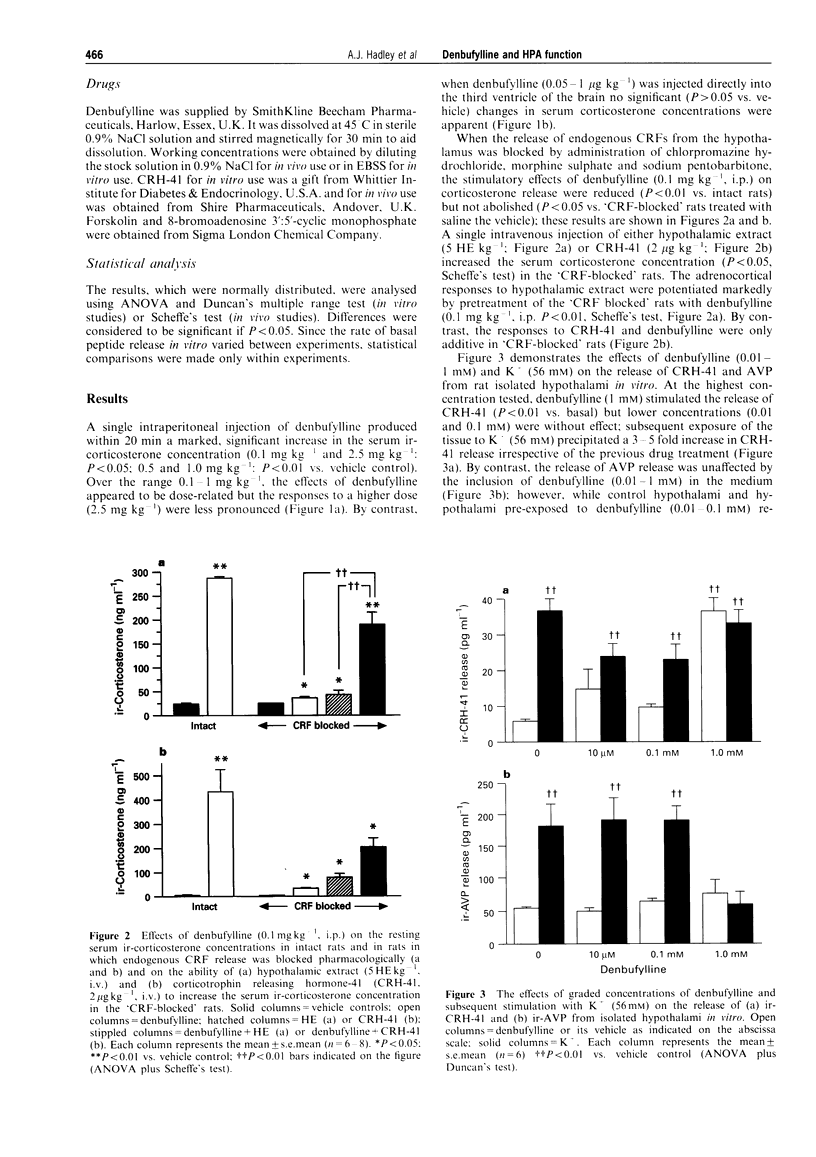
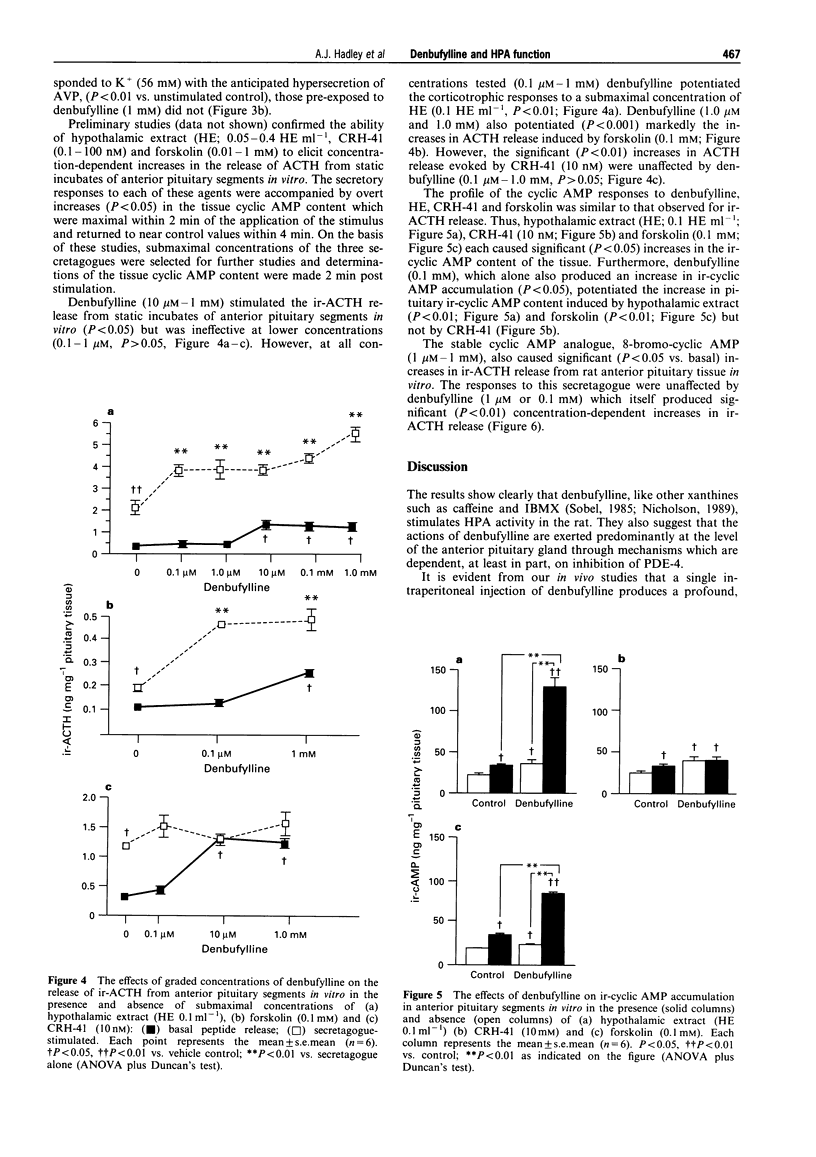
1. Preliminary studies in our laboratories showed that the synthetic xanthine analogue denbufylline, a selective type 4 phosphodiesterase (PDE-4) inhibitor, is a potent activator of the hypothalamo-pituitary-adrenal (HPA) axis when given orally to adult male rats. This paper describes the results of experiments in which well established in vivo and in vitro models were used to (a) examine further the effects of denbufylline on HPA function and (b) identify the site and mode of action of the drug within the axis. 2. In vivo, administration of denbufylline (0.1-2.5 mg kg-1, i.p.) produced a significant increase in the serum corticosterone concentration; maximal responses were attained at a dose of 1.0 mg kg-1 (P < 0.01 vs. vehicle control, Scheffe's test). However, when denbufylline was administered by intracerebroventricular injection (0.05-1 micrograms kg-1) it failed to influence significantly the serum corticosterone concentration (P > 0.05 vs. vehicle control, Scheffe's test). The adrenocortical responses to peripheral injections of denbufylline (1 mg kg-1, i.p.) were reduced in rats in which the secretion of endogenous corticotrophin releasing factors (CRFs) from the hypothalamus was blocked pharmacologically (P < 0.01 vs. controls, Scheffe's test). However, denbufylline (0.1 mg kg-1, i.p.) potentiated the significant (P < 0.01) increases in serum corticosterone concentration provoked in "CRF blocked rats' by hypothalamic extract (5 hypothalamic extracts kg-1, i.v.) although it failed to influence (P > 0.05) the relatively moderate increases in corticosterone secretion evoked by CRH-41 (2 mg kg-1, i.v.). 3. In vitro, denbufylline (0.01-1 mM) evoked small but significant (P < 0.05) increases in the release of ACTH from rat anterior pituitary segments; furthermore, at these and lower concentrations (0.01 microM-1 mM), it potentiated the adrenocorticotrophic responses to sub-maximal concentrations of hypothalamic extract (P < 0.01) and forskolin (0.1 mM, P < 0.01) but not those to CRH-41 (10 nM) or 8-bromo-cyclic AMP (1-100 microM). In addition, denbufyline (0.1 mM) increased the anterior pituitary cyclic AMP content (P < 0.05) and potentiated the rises in tissue content of the cyclic nucleotide induced by hypothalamic extract (0.1 hypothalamic equivalents ml-1, P < 0.01) and forskolin (0.1 mM, P < 0.01) but not by CRH-41 (10 nM, P < 0.05). By contrast, denbufylline (1 microM-1 mM) failed to influence the release of AVP from rat isolated hypothalami and stimulated the secretion of CRH-41 (P < 0.01) release only at the highest concentration tested (1 mM). 4. The results suggest that the stimulatory actions of denbufylline on the hypothalamo-pituitary-adrenocortical axis are exerted predominantly at the level of the anterior pituitary gland and that they may be attributed, at least in part, to inhibition of type 4 phosphodiesterase enzymes.
Full text
PDF

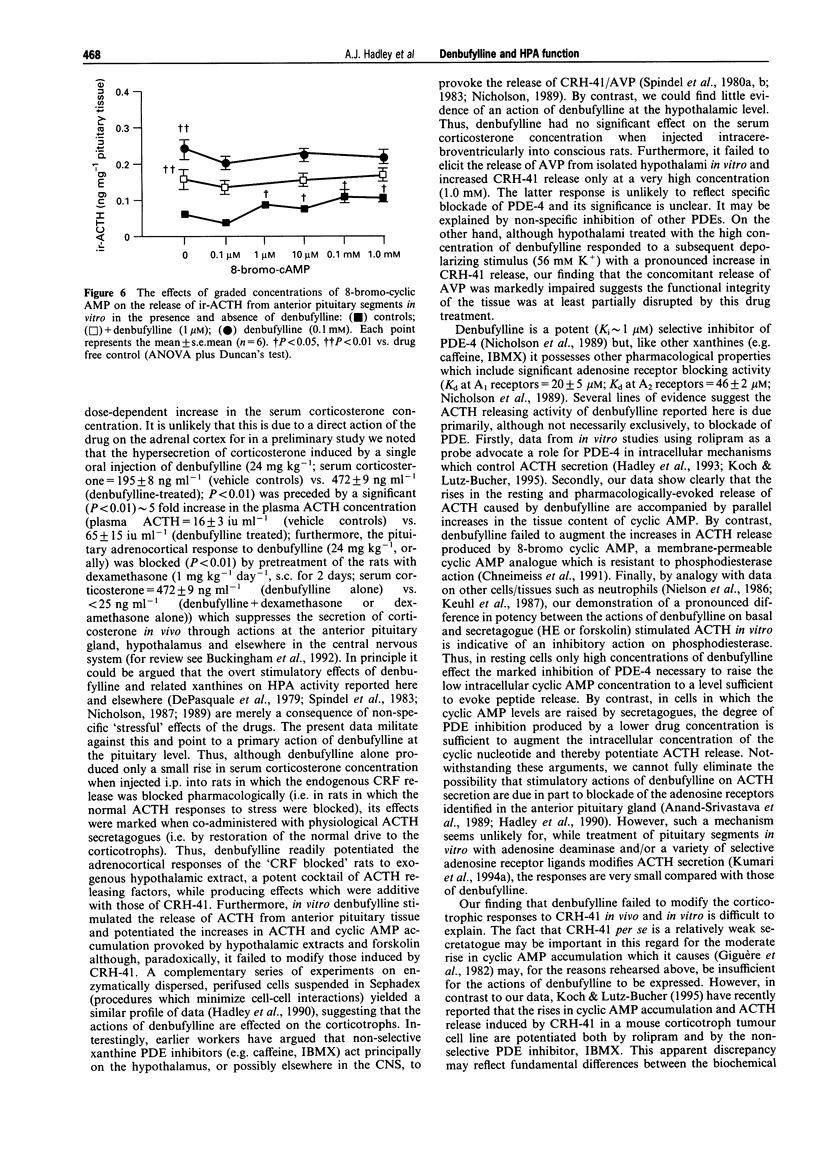


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Harwood J. P., Wilson J. X., Morell J., Brown J. H., Catt K. J. Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. J Biol Chem. 1983 Jul 10;258(13):8039–8045. [PubMed] [Google Scholar]
- Al-Dujaili E. A., Williams B. C., Edwards C. R. The development and application of a direct radioimmunoassay for corticosterone. Steroids. 1981 Feb;37(2):157–176. doi: 10.1016/s0039-128x(81)80015-3. [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava M. B., Cantin M., Gutkowska J. Adenosine regulates the release of adrenocorticotropic hormone (ACTH) from cultured anterior pituitary cells. Mol Cell Biochem. 1989 Aug 15;89(1):21–28. doi: 10.1007/BF00228276. [DOI] [PubMed] [Google Scholar]
- Arimura A., Saito T., Schally A. V. Assays for corticotropin-releasing factor (CRF) using rats treated with morphine, chlorpromazine, dexamethasone and Nembutal. Endocrinology. 1967 Aug;81(2):235–245. doi: 10.1210/endo-81-2-235. [DOI] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bentley J. K., Beavo J. A. Regulation and function of cyclic nucleotides. Curr Opin Cell Biol. 1992 Apr;4(2):233–240. doi: 10.1016/0955-0674(92)90038-e. [DOI] [PubMed] [Google Scholar]
- Bolger G., Michaeli T., Martins T., St John T., Steiner B., Rodgers L., Riggs M., Wigler M., Ferguson K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993 Oct;13(10):6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham J. C., Hodges J. R. The use of corticotrophin production by adenohypophysial tissue in vitro for the detection and estimation of potential corticotrophin releasing factors. J Endocrinol. 1977 Feb;72(2):187–193. doi: 10.1677/joe.0.0720187. [DOI] [PubMed] [Google Scholar]
- Buckingham J. C. Inhibition of corticotrophin releasing factor secretion in the pentobarbitone-morphine-treated rat. Eur J Pharmacol. 1984 Feb 17;98(2):211–221. doi: 10.1016/0014-2999(84)90592-2. [DOI] [PubMed] [Google Scholar]
- Chneiweiss H., Cordier J., Glowinski J. Cyclic AMP accumulation induces a rapid desensitization of the cyclic AMP-dependent protein kinase in mouse striatal neurons. J Neurochem. 1991 Nov;57(5):1708–1715. doi: 10.1111/j.1471-4159.1991.tb06371.x. [DOI] [PubMed] [Google Scholar]
- Conti M., Jin S. L., Monaco L., Repaske D. R., Swinnen J. V. Hormonal regulation of cyclic nucleotide phosphodiesterases. Endocr Rev. 1991 Aug;12(3):218–234. doi: 10.1210/edrv-12-3-218. [DOI] [PubMed] [Google Scholar]
- De Pasquale A., Costa G., Trovato A., Ceserani R. Effect of prostaglandins on the increased corticosterone output induced by caffeine in the rat. Prostaglandins Med. 1979 Aug;3(2):97–103. doi: 10.1016/0161-4630(79)90077-6. [DOI] [PubMed] [Google Scholar]
- Emanuel R. L., Girard D. M., Thull D. L., Majzoub J. A. Second messengers involved in the regulation of corticotropin-releasing hormone mRNA and peptide in cultured rat fetal hypothalamic primary cultures. Endocrinology. 1990 Jun;126(6):3016–3021. doi: 10.1210/endo-126-6-3016. [DOI] [PubMed] [Google Scholar]
- Giguere V., Labrie F. Vasopressin potentiates cyclic AMP accumulation and ACTH release induced by corticotropin-releasing factor (CRF) in rat anterior pituitary cells in culture. Endocrinology. 1982 Nov;111(5):1752–1754. doi: 10.1210/endo-111-5-1752. [DOI] [PubMed] [Google Scholar]
- Hillhouse E. W., Milton N. G. Effect of acetylcholine and 5-hydroxytryptamine on the secretion of corticotrophin-releasing factor-41 and arginine vasopressin from the rat hypothalamus in vitro. J Endocrinol. 1989 Sep;122(3):713–718. doi: 10.1677/joe.0.1220713. [DOI] [PubMed] [Google Scholar]
- Koch B., Lutz-Bucher B. Multifactorial regulation of pituitary adenylate cyclase-activating polypeptide (PACAP)-induced production of cyclic AMP in ATT-20 corticotrophs: major involvement of Rolipram-sensitive and insensitive phosphodiesterases. Mol Cell Endocrinol. 1995 Jul;112(1):27–34. doi: 10.1016/0303-7207(95)03583-s. [DOI] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Zanetti M. E., Soderman D. D., Miller D. K., Ham E. A. Cyclic AMP-dependent regulation of lipid mediators in white cells. A unifying concept for explaining the efficacy of theophylline in asthma. Am Rev Respir Dis. 1987 Jul;136(1):210–213. doi: 10.1164/ajrccm/136.1.210. [DOI] [PubMed] [Google Scholar]
- Labrie F., Gagné B., Lefèvre G. Corticotropin-releasing factor stimulants adenylate cyclase activity in the anterior pituitary gland. Life Sci. 1982 Sep 13;31(11):1117–1121. doi: 10.1016/0024-3205(82)90085-6. [DOI] [PubMed] [Google Scholar]
- Labrie F., Veilleux R., Lefevre G., Coy D. H., Sueiras-Diaz J., Schally A. V. Corticotropin-releasing factor stimulates accumulation of adenosine 3', 5'-monophosphate in rat pituitary corticotrophs. Science. 1982 May 28;216(4549):1007–1008. doi: 10.1126/science.6281886. [DOI] [PubMed] [Google Scholar]
- Loxley H. D., Cowell A. M., Flower R. J., Buckingham J. C. Effects of lipocortin 1 and dexamethasone on the secretion of corticotrophin-releasing factors in the rat: in vitro and in vivo studies. J Neuroendocrinol. 1993 Feb;5(1):51–61. doi: 10.1111/j.1365-2826.1993.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Loxley H. D., Cowell A. M., Flower R. J., Buckingham J. C. Modulation of the hypothalamo-pituitary-adrenocortical responses to cytokines in the rat by lipocortin 1 and glucocorticoids: a role for lipocortin 1 in the feedback inhibition of CRF-41 release? Neuroendocrinology. 1993 May;57(5):801–814. doi: 10.1159/000126439. [DOI] [PubMed] [Google Scholar]
- Michaeli T., Bloom T. J., Martins T., Loughney K., Ferguson K., Riggs M., Rodgers L., Beavo J. A., Wigler M. Isolation and characterization of a previously undetected human cAMP phosphodiesterase by complementation of cAMP phosphodiesterase-deficient Saccharomyces cerevisiae. J Biol Chem. 1993 Jun 15;268(17):12925–12932. [PubMed] [Google Scholar]
- Negro-Vilar A., Sanchez-Franco F., Kwiatkowski M., Samson W. K. Failure to detect radioimmunoassayable arginine vasotocin in mammalian pineals. Brain Res Bull. 1979 Nov-Dec;4(6):789–792. doi: 10.1016/0361-9230(79)90013-3. [DOI] [PubMed] [Google Scholar]
- Nicholson C. D., Jackman S. A., Wilke R. The ability of denbufylline to inhibit cyclic nucleotide phosphodiesterase and its affinity for adenosine receptors and the adenosine re-uptake site. Br J Pharmacol. 1989 Jul;97(3):889–897. doi: 10.1111/j.1476-5381.1989.tb12029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. D., Shahid M. Inhibitors of cyclic nucleotide phosphodiesterase isoenzymes--their potential utility in the therapy of asthma. Pulm Pharmacol. 1994 Feb;7(1):1–17. doi: 10.1006/pulp.1994.1001. [DOI] [PubMed] [Google Scholar]
- Nicholson S. A. Stimulatory effect of caffeine on the hypothalamo-pituitary-adrenocortical axis in the rat. J Endocrinol. 1989 Aug;122(2):535–543. doi: 10.1677/joe.0.1220535. [DOI] [PubMed] [Google Scholar]
- Nielson C. P., Crowley J. J., Cusack B. J., Vestal R. E. Therapeutic concentrations of theophylline and enprofylline potentiate catecholamine effects and inhibit leukocyte activation. J Allergy Clin Immunol. 1986 Oct;78(4 Pt 1):660–667. doi: 10.1016/0091-6749(86)90086-2. [DOI] [PubMed] [Google Scholar]
- Rees L. H., Cook D. M., Kendall J. W., Allen C. F., Kramer R. M., Ratcliffe J. G., Knight R. A. A radioimmunoassay for rat plasma ACTH. Endocrinology. 1971 Jul;89(1):254–261. doi: 10.1210/endo-89-1-254. [DOI] [PubMed] [Google Scholar]
- Repaske D. R., Swinnen J. V., Jin S. L., Van Wyk J. J., Conti M. A polymerase chain reaction strategy to identify and clone cyclic nucleotide phosphodiesterase cDNAs. Molecular cloning of the cDNA encoding the 63-kDa calmodulin-dependent phosphodiesterase. J Biol Chem. 1992 Sep 15;267(26):18683–18688. [PubMed] [Google Scholar]
- Seasholtz A. F., Thompson R. C., Douglass J. O. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol. 1988 Dec;2(12):1311–1319. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- Sette C., Iona S., Conti M. The short-term activation of a rolipram-sensitive, cAMP-specific phosphodiesterase by thyroid-stimulating hormone in thyroid FRTL-5 cells is mediated by a cAMP-dependent phosphorylation. J Biol Chem. 1994 Mar 25;269(12):9245–9252. [PubMed] [Google Scholar]
- Sette C., Vicini E., Conti M. Modulation of cellular responses by hormones: role of cAMP specific, rolipram-sensitive phosphodiesterases. Mol Cell Endocrinol. 1994 Apr;100(1-2):75–79. doi: 10.1016/0303-7207(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Sobel D. O. Role of cyclic AMP in corticotropin releasing factor mediated ACTH release. Peptides. 1985 Jul-Aug;6(4):591–595. doi: 10.1016/0196-9781(85)90158-5. [DOI] [PubMed] [Google Scholar]
- Spindel E., Arnold M., Cusack B., Wurtman R. J. Effects of caffeine on anterior pituitary and thyroid function in the rat. J Pharmacol Exp Ther. 1980 Jul;214(1):58–62. [PubMed] [Google Scholar]
- Spindel E., Griffith L., Wurtman R. J. Neuroendocrine effects of caffeine. II. Effects on thyrotropin and corticosterone secretion. J Pharmacol Exp Ther. 1983 May;225(2):346–350. [PubMed] [Google Scholar]
- Sullivan P., Bekir S., Jaffar Z., Page C., Jeffery P., Costello J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994 Apr 23;343(8904):1006–1008. doi: 10.1016/s0140-6736(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Todd K., Lightman S. L. Vasopressin activation of phosphatidylinositol metabolism in rat anterior pituitary in vitro and its modification by changes in the hypothalamo-pituitary-adrenal axis. Neuroendocrinology. 1987 Mar;45(3):212–218. doi: 10.1159/000124728. [DOI] [PubMed] [Google Scholar]