Abstract
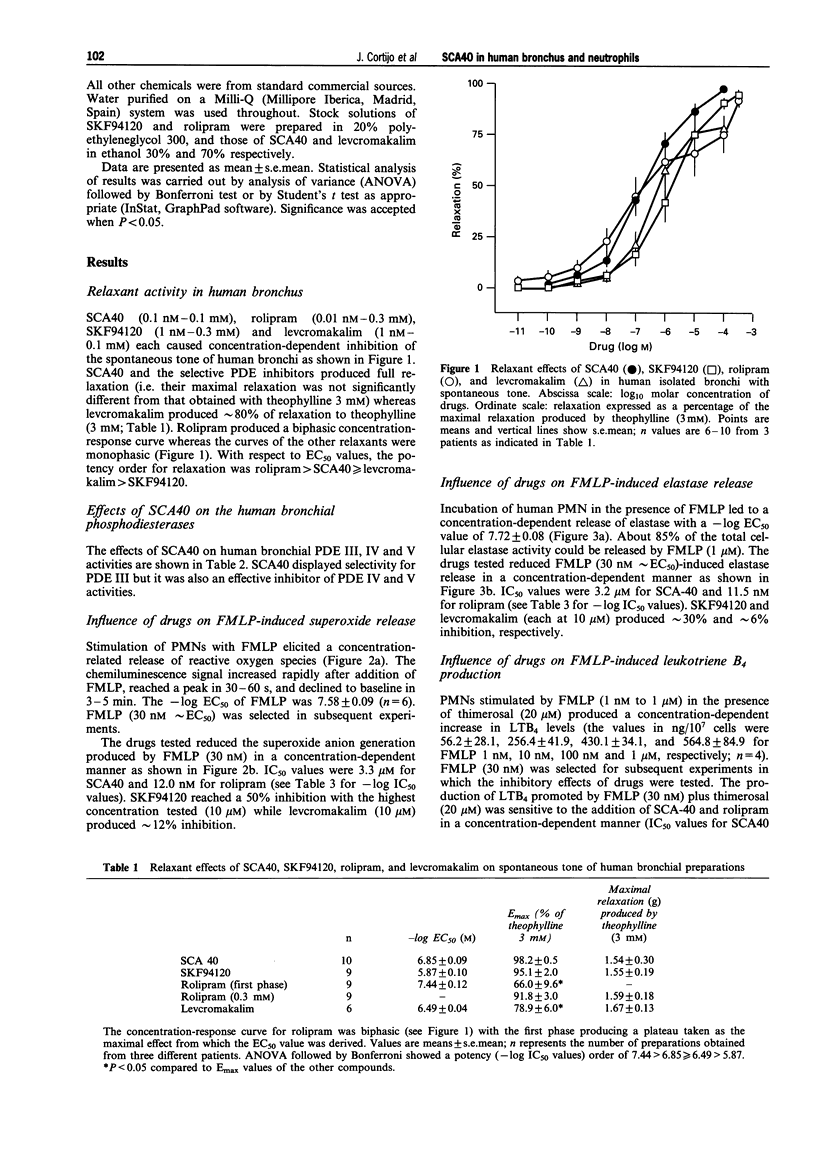
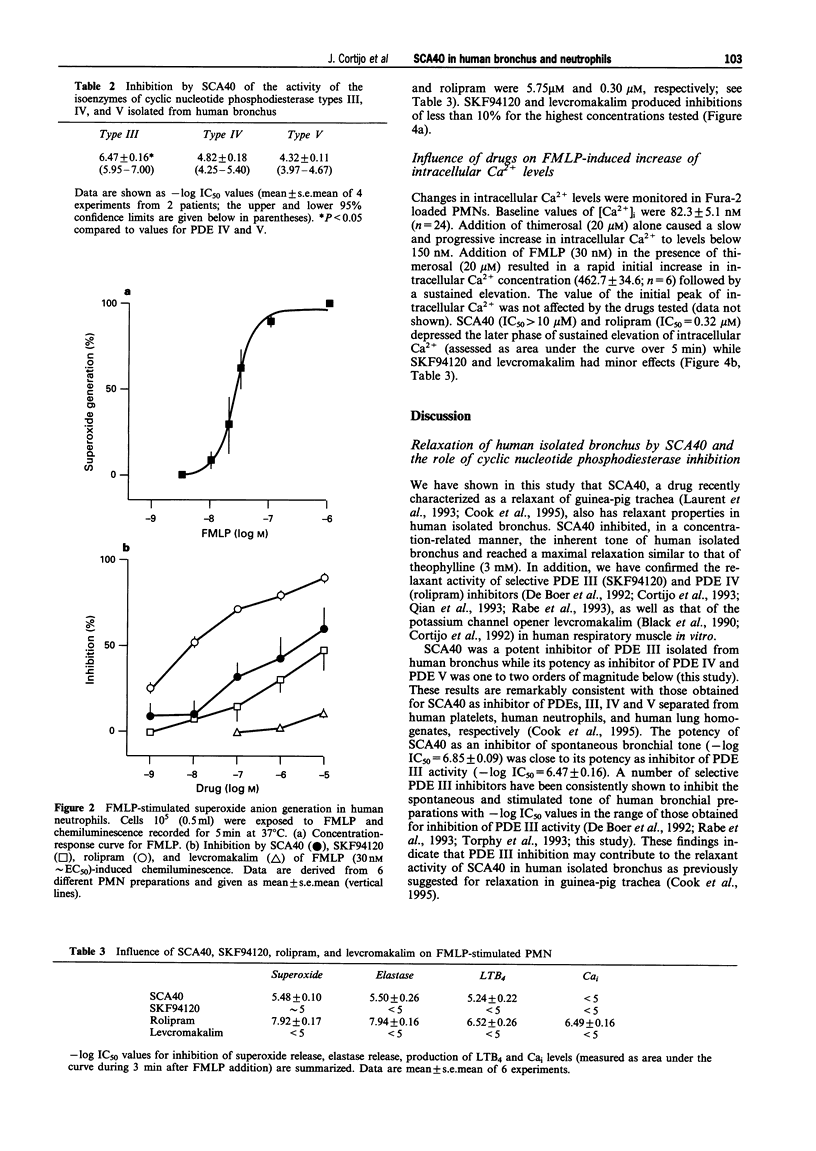
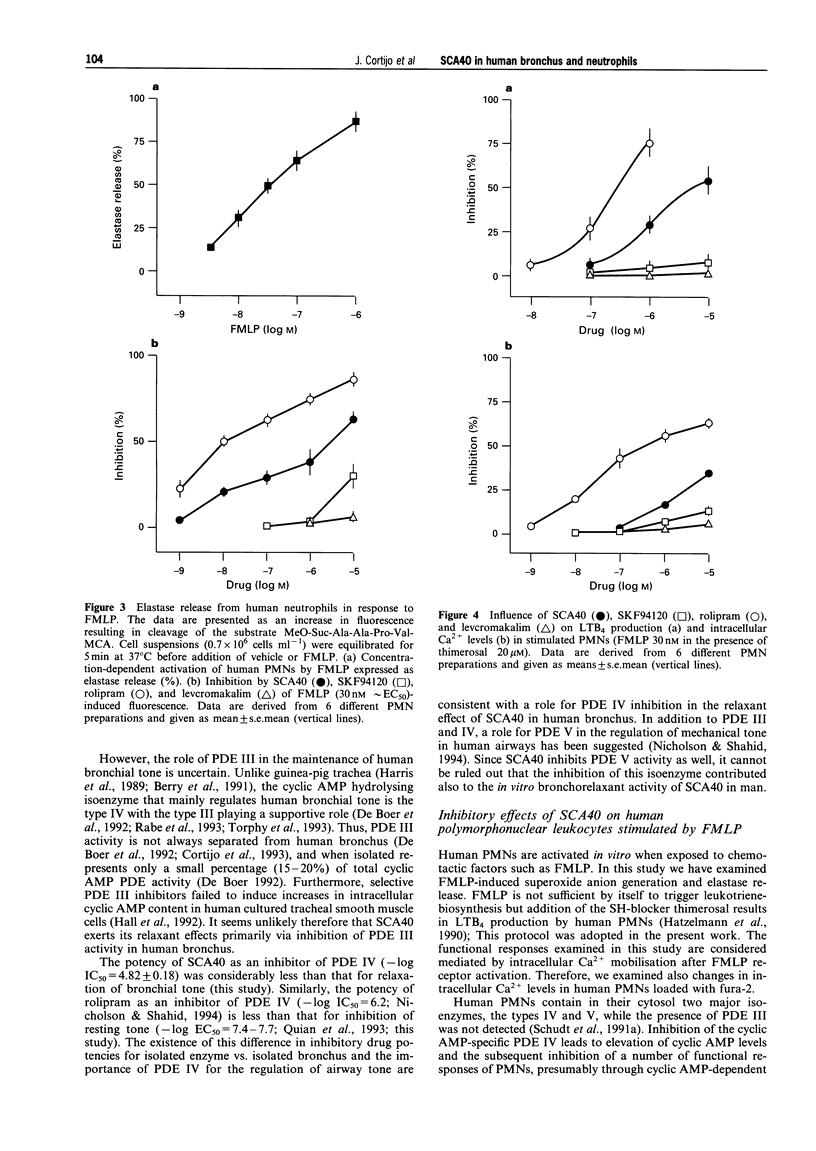
1. SCA40 (0.1 nM-0.1 mM) produced concentration-dependent suppression of the spontaneous tone of human isolated bronchus (-log EC50 = 6.85 +/- 0.09; n = 10) and reached a maximal relaxation similar to that of theophylline (3 mM). The potency (-log EC50 values) of SCA40 compared to other relaxants was rolipram (7.44 +/- 0.12; n = 9) > SCA40 > or = levcromakalim (6.49 +/- 0.04; n = 6) > SKF94120 (5.87 +/- 0.10; n = 9). 2. When tested against the activity of the isoenzymes of cyclic nucleotide phosphodiesterase (PDE) isolated from human bronchus, SCA40 proved highly potent against PDE III (-log IC50 = 6.47 +/- 0.16; n = 4). It was markedly less potent against PDE IV (4.82 +/- 0.18; n = 4) and PDE V (4.32 +/- 0.11; n = 4). 3. Human polymorphonuclear leukocytes (PMNs) stimulated with N-formylmethionyl-leucyl-phenylalanine (FMLP) produced a concentration-dependent superoxide anion generation and elastase release. SCA40 (1 nM-10 microM) produced a concentration-related inhibition of FMLP (30 nM approximately EC50)-induced superoxide production (-log IC50 = 5.48 +/- 0.10; n = 6) and elastase release (-log IC50 = 5.50 +/- 0.26; n = 6). Rolipram was an effective inhibitor of superoxide generation and elastase release (-log IC50 values approximately 8) while SKF94120 and levcromakalim were scarcely effective. 4. FMLP (30 nM) and thimerosal (20 microM) induced leukotriene B4 production and elevation of intracellular calcium concentration in human PMNs. The production of leukotriene B4 was inhibited by SCA40 in a concentration-related manner (-log IC50 = 5.94 +/- 0.22; n = 6) but SCA40 was less effective against the elevation of intracellular calcium. Rolipram was an effective inhibitor of leukotriene B4 synthesis (-log IC50 approximately 7) and intracellular calcium elevation (-log IC50 approximately 6) while SKF94120 and levcromakalim were scarcely effective. 5. It is concluded that SCA40 is an effective inhibitor of the inherent tone of human isolated bronchus. The bronchodilatation produced by SCA40 appears mainly related to PDE inhibition since the potency of SCA40 as a relaxant of human isolated bronchus was found to be close to its potency as inhibitor of PDE III activity isolated from human bronchus. In addition, SCA40 exhibited inhibitory effects on human PMN function stimulated by FMLP. These effects may be related to the ability of SCA40 to inhibit PDE IV from human PMNs while the contribution of PDE V inhibition is uncertain. We found no evidence of a role for levcromakalim-sensitive plasmalemmal K+-channels in human PMNs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Black J. L., Armour C. L., Johnson P. R., Alouan L. A., Barnes P. J. The action of a potassium channel activator, BRL 38227 (lemakalim), on human airway smooth muscle. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1384–1389. doi: 10.1164/ajrccm/142.6_Pt_1.1384. [DOI] [PubMed] [Google Scholar]
- Bonnet P. A., Michel A., Laurent F., Sablayrolles C., Rechencq E., Mani J. C., Boucard M., Chapat J. P. Synthesis and antibronchospastic activity of 8-alkoxy- and 8-(alkylamino)imidazo[1,2-a]pyrazines. J Med Chem. 1992 Sep 4;35(18):3353–3358. doi: 10.1021/jm00096a008. [DOI] [PubMed] [Google Scholar]
- Buckle D. R. Prospects for potassium channel activators in the treatment of airways obstruction. Pulm Pharmacol. 1993 Sep;6(3):161–169. doi: 10.1006/pulp.1993.1022. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cook S. J., Archer K., Martin A., Buchheit K. H., Fozard J. R., Müller T., Miller A. J., Elliott K. R., Foster R. W., Small R. C. Further analysis of the mechanisms underlying the tracheal relaxant action of SCA40. Br J Pharmacol. 1995 Jan;114(1):143–151. doi: 10.1111/j.1476-5381.1995.tb14918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortijo J., Bou J., Beleta J., Cardelús I., Llenas J., Morcillo E., Gristwood R. W. Investigation into the role of phosphodiesterase IV in bronchorelaxation, including studies with human bronchus. Br J Pharmacol. 1993 Feb;108(2):562–568. doi: 10.1111/j.1476-5381.1993.tb12841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortijo J., Sarriá B., Pedrós C., Perpiñ M., Paris F., Morcillo E. The relaxant effects of cromakalim (BRL 34915) on human isolated airway smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1992 Oct;346(4):462–468. doi: 10.1007/BF00171091. [DOI] [PubMed] [Google Scholar]
- De Vries G. W., Amdahl L. D., Kramer K. D., Wheeler L. A. Inhibition by manoalide of fMLP-stimulated elastase release from human neutrophils. Biochem Pharmacol. 1990 Dec 1;40(11):2487–2490. doi: 10.1016/0006-2952(90)90090-8. [DOI] [PubMed] [Google Scholar]
- Ervens J., Schultz G., Seifert R. Differential inhibition and potentiation of chemoattractant-induced superoxide formation in human neutrophils by the cell-permeant analogue of cyclic GMP, N2,2'-O-dibutyryl guanosine 3':5'-cyclic monophosphate. Naunyn Schmiedebergs Arch Pharmacol. 1991 Apr;343(4):370–376. doi: 10.1007/BF00179041. [DOI] [PubMed] [Google Scholar]
- Feth F., Rascher W., Michel M. C. Neuropeptide Y (NPY) receptors in HEL cells: comparison of binding and functional parameters for full and partial agonists and a non-peptide antagonist. Br J Pharmacol. 1992 Jan;105(1):71–76. doi: 10.1111/j.1476-5381.1992.tb14212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giembycz M. A. Could isoenzyme-selective phosphodiesterase inhibitors render bronchodilator therapy redundant in the treatment of bronchial asthma? Biochem Pharmacol. 1992 May 28;43(10):2041–2051. doi: 10.1016/0006-2952(92)90160-k. [DOI] [PubMed] [Google Scholar]
- Gristwood R. W., Beleta J., Bou J., Cardelús I., Fernández A. G., Llenas J., Berga P. Studies on the cardiac actions of flosequinan in vitro. Br J Pharmacol. 1992 Apr;105(4):985–991. doi: 10.1111/j.1476-5381.1992.tb09089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamilton T. C., Weston A. H. Cromakalim, nicorandil and pinacidil: novel drugs which open potassium channels in smooth muscle. Gen Pharmacol. 1989;20(1):1–9. doi: 10.1016/0306-3623(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Connell M. J., Ferguson E. W., Wallace A. M., Gordon R. J., Pagani E. D., Silver P. J. Role of low Km cyclic AMP phosphodiesterase inhibition in tracheal relaxation and bronchodilation in the guinea pig. J Pharmacol Exp Ther. 1989 Oct;251(1):199–206. [PubMed] [Google Scholar]
- Hatzelmann A., Haurand M., Ullrich V. Involvement of calcium in the thimerosal-stimulated formation of leukotriene by fMLP in human polymorphonuclear leukocytes. Biochem Pharmacol. 1990 Feb 1;39(3):559–567. doi: 10.1016/0006-2952(90)90064-r. [DOI] [PubMed] [Google Scholar]
- Herráez A. ELEASY: a program for processing experimental enzymoimmunoassay data. J Immunol Methods. 1993 Dec 3;166(2):297–298. doi: 10.1016/0022-1759(93)90373-f. [DOI] [PubMed] [Google Scholar]
- Laurent F., Michel A., Bonnet P. A., Chapat J. P., Boucard M. Evaluation of the relaxant effects of SCA40, a novel charybdotoxin-sensitive potassium channel opener, in guinea-pig isolated trachealis. Br J Pharmacol. 1993 Mar;108(3):622–626. doi: 10.1111/j.1476-5381.1993.tb12851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan S., Sheridan R. D., Chilvers E. R., Patmore L. A comparison of the effects of SCA40, NS 004 and NS 1619 on large conductance Ca(2+)-activated K+ channels in bovine tracheal smooth muscle cells in culture. Br J Pharmacol. 1995 Sep;116(1):1656–1660. doi: 10.1111/j.1476-5381.1995.tb16387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A., Laurent F., Bompart J., Hadj-Kaddour K., Chapat J. P., Boucard M., Bonnet P. A. Cardiovascular effects of SCA40, a novel potassium channel opener, in rats. Br J Pharmacol. 1993 Nov;110(3):1031–1036. doi: 10.1111/j.1476-5381.1993.tb13917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M., Inoue M., Tokumaru S., Kogo H. Enhancing and inhibitory effects of nitric oxide on superoxide anion generation in human polymorphonuclear leukocytes. Br J Pharmacol. 1995 Aug;115(7):1302–1306. doi: 10.1111/j.1476-5381.1995.tb15040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J. K+ channel openers and suppression of airway hyperreactivity. Trends Pharmacol Sci. 1994 Dec;15(12):463–468. doi: 10.1016/0165-6147(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Mueller H., Sklar L. A. Coupling of antagonistic signalling pathways in modulation of neutrophil function. J Cell Biochem. 1989 Jul;40(3):287–294. doi: 10.1002/jcb.240400305. [DOI] [PubMed] [Google Scholar]
- Nicholson C. D., Jackman S. A., Wilke R. The ability of denbufylline to inhibit cyclic nucleotide phosphodiesterase and its affinity for adenosine receptors and the adenosine re-uptake site. Br J Pharmacol. 1989 Jul;97(3):889–897. doi: 10.1111/j.1476-5381.1989.tb12029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. D., Shahid M. Inhibitors of cyclic nucleotide phosphodiesterase isoenzymes--their potential utility in the therapy of asthma. Pulm Pharmacol. 1994 Feb;7(1):1–17. doi: 10.1006/pulp.1994.1001. [DOI] [PubMed] [Google Scholar]
- Pieper G. M., Gross G. J. EMD 52692 (bimakalim), a new potassium channel opener, attenuates luminol-enhanced chemiluminescence and superoxide anion radical formation by zymosan-activated polymorphonuclear leukocytes. Immunopharmacology. 1992 May-Jun;23(3):191–197. doi: 10.1016/0162-3109(92)90025-8. [DOI] [PubMed] [Google Scholar]
- Qian Y., Naline E., Karlsson J. A., Raeburn D., Advenier C. Effects of rolipram and siguazodan on the human isolated bronchus and their interaction with isoprenaline and sodium nitroprusside. Br J Pharmacol. 1993 Jul;109(3):774–778. doi: 10.1111/j.1476-5381.1993.tb13641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe K. F., Tenor H., Dent G., Schudt C., Liebig S., Magnussen H. Phosphodiesterase isozymes modulating inherent tone in human airways: identification and characterization. Am J Physiol. 1993 May;264(5 Pt 1):L458–L464. doi: 10.1152/ajplung.1993.264.5.L458. [DOI] [PubMed] [Google Scholar]
- Reeves M. L., Leigh B. K., England P. J. The identification of a new cyclic nucleotide phosphodiesterase activity in human and guinea-pig cardiac ventricle. Implications for the mechanism of action of selective phosphodiesterase inhibitors. Biochem J. 1987 Jan 15;241(2):535–541. doi: 10.1042/bj2410535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Della Bianca V., Grzeskowiak M., De Togni P., Cabrini G. Relationships between phosphoinositide metabolism, Ca2+ changes and respiratory burst in formyl-methionyl-leucyl-phenylalanine-stimulated human neutrophils. The breakdown of phosphoinositides is not involved in the rise of cytosolic free Ca2+. FEBS Lett. 1985 Feb 25;181(2):253–258. doi: 10.1016/0014-5793(85)80270-2. [DOI] [PubMed] [Google Scholar]
- Schudt C., Winder S., Forderkunz S., Hatzelmann A., Ullrich V. Influence of selective phosphodiesterase inhibitors on human neutrophil functions and levels of cAMP and Cai. Naunyn Schmiedebergs Arch Pharmacol. 1991 Dec;344(6):682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- Schudt C., Winder S., Müller B., Ukena D. Zardaverine as a selective inhibitor of phosphodiesterase isozymes. Biochem Pharmacol. 1991 Jun 21;42(1):153–162. doi: 10.1016/0006-2952(91)90694-z. [DOI] [PubMed] [Google Scholar]
- Shahid M., van Amsterdam R. G., de Boer J., ten Berge R. E., Nicholson C. D., Zaagsma J. The presence of five cyclic nucleotide phosphodiesterase isoenzyme activities in bovine tracheal smooth muscle and the functional effects of selective inhibitors. Br J Pharmacol. 1991 Oct;104(2):471–477. doi: 10.1111/j.1476-5381.1991.tb12453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., McNeil V. M., Jesaitis A. J., Painter R. G., Cochrane C. G. A continuous, spectroscopic analysis of the kinetics of elastase secretion by neutrophils. The dependence of secretion upon receptor occupancy. J Biol Chem. 1982 May 25;257(10):5471–5475. [PubMed] [Google Scholar]
- Small R. C., Chiu P., Cook S. J., Foster R. W., Isaac L. Beta-adrenoceptor agonists in bronchial asthma: role of k+-channel opening in mediating their bronchodilator effects. Clin Exp Allergy. 1993 Oct;23(10):802–811. doi: 10.1111/j.1365-2222.1993.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Thompson N. T., Bonser R. W., Tateson J. E., Spacey G. D., Randall R. W., Hodson H. F., Garland L. G. A quantitative investigation into the dependence of Ca2+ mobilisation on changes in inositol 1,4,5-trisphosphate levels in the stimulated neutrophil. Br J Pharmacol. 1991 Jun;103(2):1592–1596. doi: 10.1111/j.1476-5381.1991.tb09832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy T. J., Undem B. J., Cieslinski L. B., Luttmann M. A., Reeves M. L., Hay D. W. Identification, characterization and functional role of phosphodiesterase isozymes in human airway smooth muscle. J Pharmacol Exp Ther. 1993 Jun;265(3):1213–1223. [PubMed] [Google Scholar]
- de Boer J., Philpott A. J., van Amsterdam R. G., Shahid M., Zaagsma J., Nicholson C. D. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br J Pharmacol. 1992 Aug;106(4):1028–1034. doi: 10.1111/j.1476-5381.1992.tb14451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


