Abstract
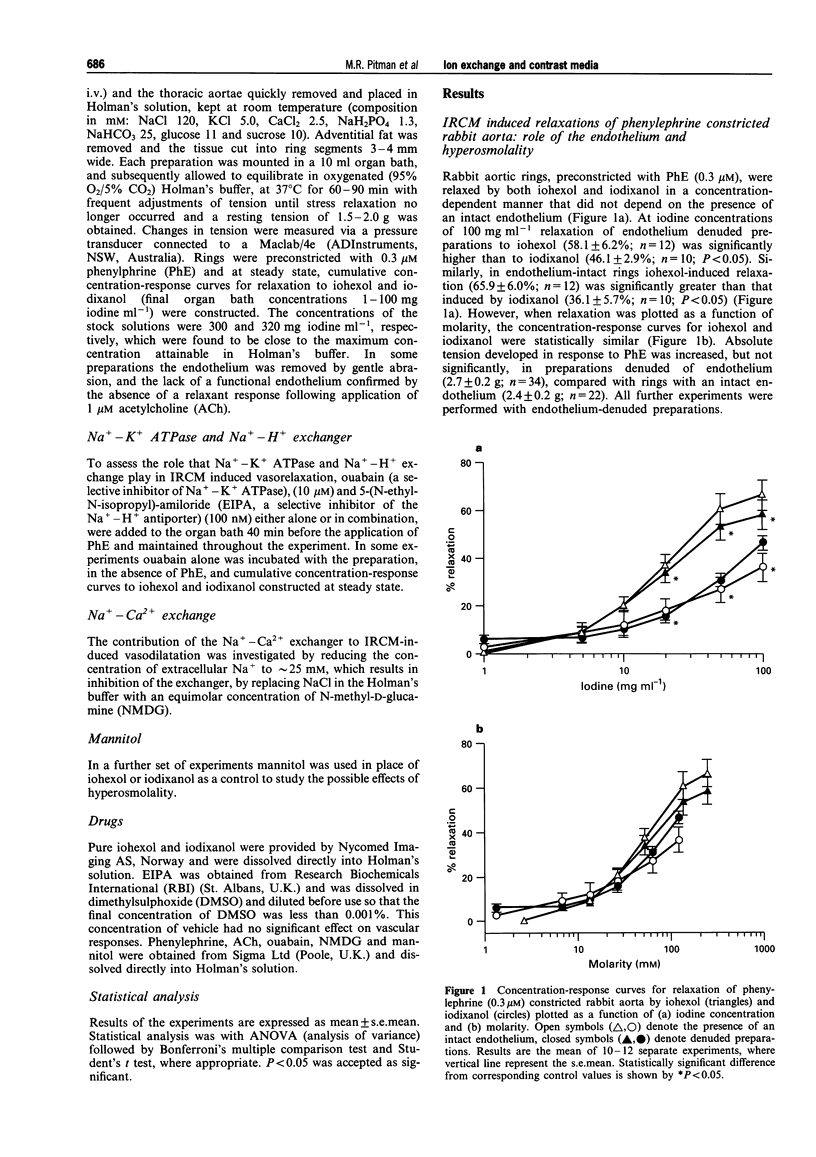
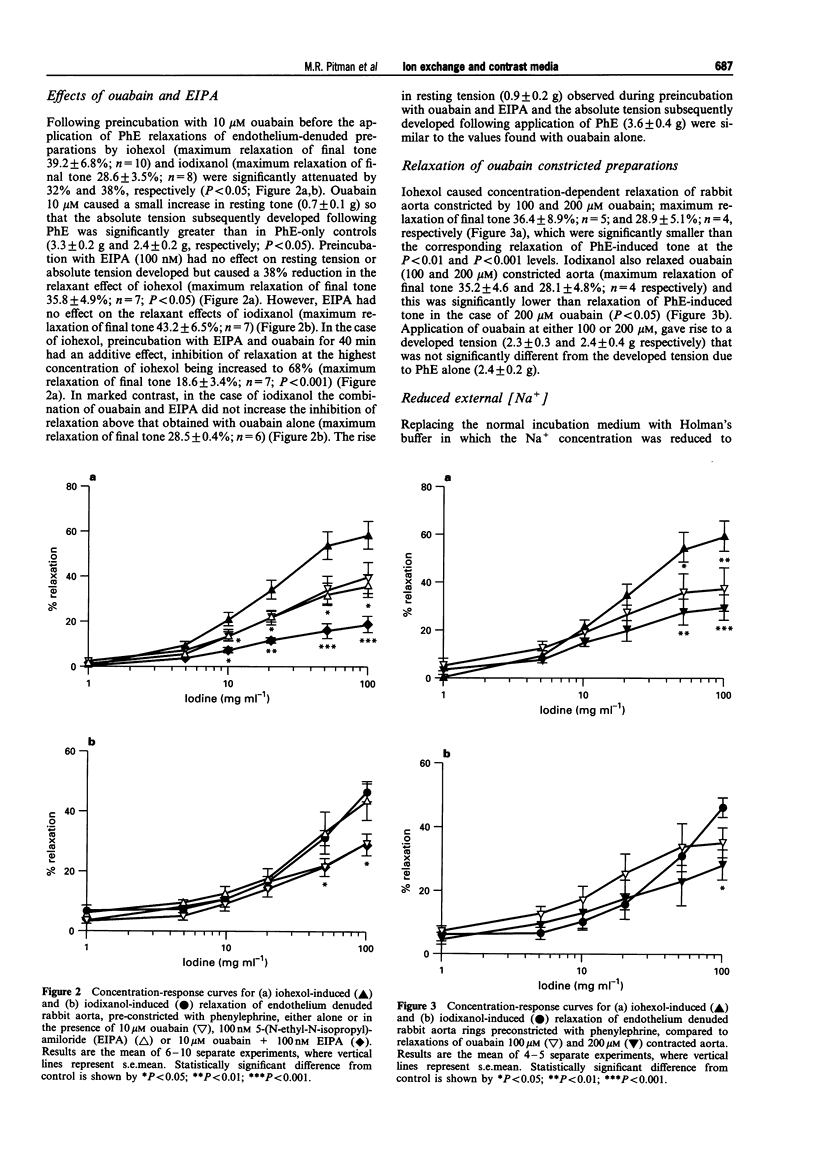
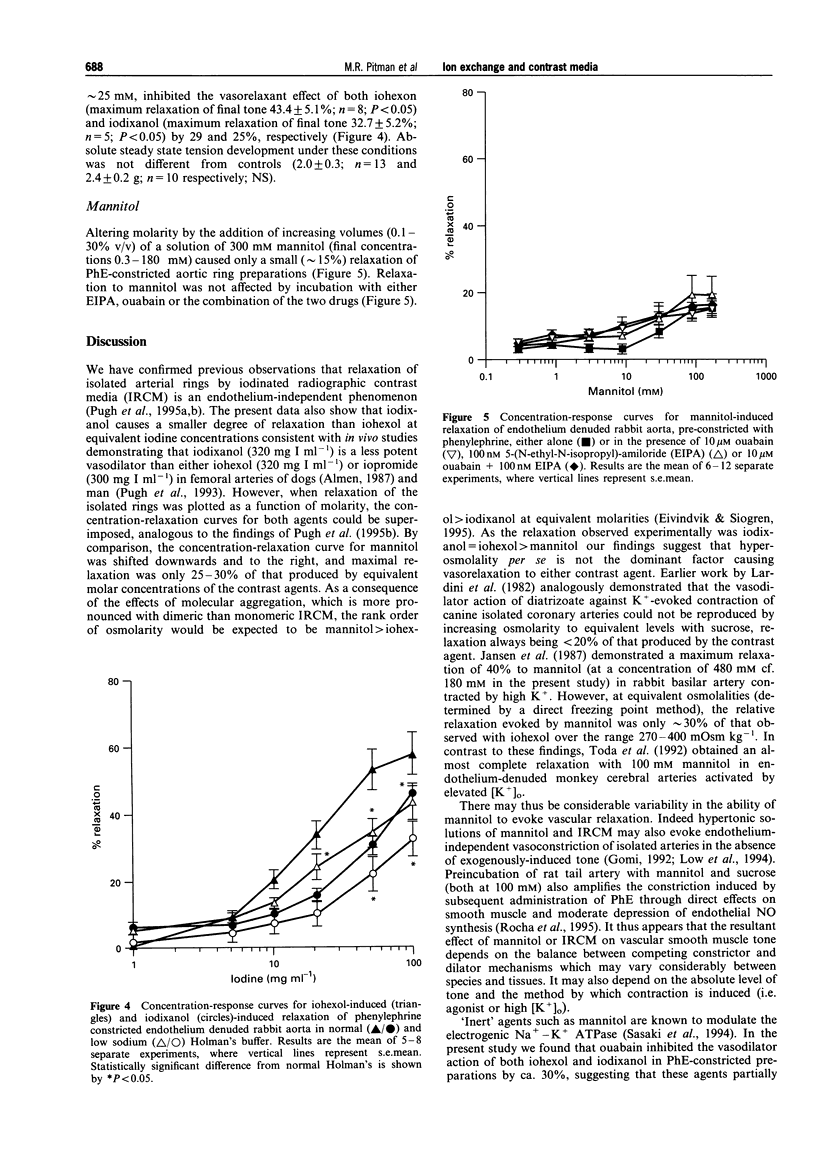
1. We have used rings of rabbit thoracic aorta to investigate the vasorelaxant properties of two different classes of non-ionic iodinated radiographic contrast media (IRCM) and the mechanisms, underlying their mode of action. Iohexol (a triiodinated monomer) was compared with iodixanol (a hexaiodinated dimer). 2. Iohexol and iodixanol both relaxed phenylephrine (0.3 microM) constricted rabbit aorta in a concentration-dependent manner that did not depend on the presence of an intact endothelium. When expressed as a function of iodine concentration, iodixanol caused significantly less relaxation than iohexol. However, the extent of relaxation was similar for both IRCM when expressed on a molar basis. Furthermore, increasing the molarity of the buffer to comparable levels with mannitol evoked only a small (approximately 15%) relaxation of phenylephrine-induced tone. 3. Ouabain (10 microM) significantly inhibited both iohexol- and iodixanol-induced relaxations by approximately 30%. 5-(N-Ethyl-N-isopropyl)-amiloride (EIPA, 100 nM) significantly inhibited iohexol-induced relaxation to the same extent as ouabain, but did not alter the vasorelaxant effect of iodixanol. Co-incubation with ouabain and EIPA had an additive effect in the case of iohexol, increasing inhibition of relaxation to approximately 60%, whereas inhibition of iodixanol-induced relaxation by the combination of ouabain plus EIPA did not differ from that of ouabain alone. 4. Replacing NaCl with N-methyl-D-glucamine (NMDG) to lower extracellular [Na+] and thereby inhibit Na(+)-Ca2+ exchange, attenuated the relaxation evoked by iohexol or by iodixanol (by approximately 25%) in each case. 5. We conclude that iohexol- and iodixanol-induced vasorelaxation in rabbit aorta is mediated through a direct action on vascular smooth muscle that is not simply a consequence of altered osmolality. It involves modulation of the Na(+)-K+ ATPase and, in the case of iohexol, Na(+)-H+ exchange. Both agents also appear to modulate Na(+)-Ca2+ exchange, through direct and/or indirect mechanisms. This is the first study to show specific pharmacological differences between monomeric and dimeric contrast media in vascular smooth muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almén T. Effects of iodixanol, iopentol, iohexol and metrizoate on femoral blood flow after injection into the femoral artery of the dog. Acta Radiol Suppl. 1987;370:69–72. [PubMed] [Google Scholar]
- Ashida T., Blaustein M. P. Regulation of cell calcium and contractility in mammalian arterial smooth muscle: the role of sodium-calcium exchange. J Physiol. 1987 Nov;392:617–635. doi: 10.1113/jphysiol.1987.sp016800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Sodium/calcium exchange and the control of contractility in cardiac muscle and vascular smooth muscle. J Cardiovasc Pharmacol. 1988;12 (Suppl 5):S56–S68. [PubMed] [Google Scholar]
- Bush W. H., Swanson D. P. Acute reactions to intravascular contrast media: types, risk factors, recognition, and specific treatment. AJR Am J Roentgenol. 1991 Dec;157(6):1153–1161. doi: 10.2214/ajr.157.6.1950858. [DOI] [PubMed] [Google Scholar]
- Danthuluri N. R., Deth R. C. Effects of intracellular alkalinization on resting and agonist-induced vascular tone. Am J Physiol. 1989 Mar;256(3 Pt 2):H867–H875. doi: 10.1152/ajpheart.1989.256.3.H867. [DOI] [PubMed] [Google Scholar]
- Dascalu A., Nevo Z., Korenstein R. Hyperosmotic activation of the Na(+)-H+ exchanger in a rat bone cell line: temperature dependence and activation pathways. J Physiol. 1992 Oct;456:503–518. doi: 10.1113/jphysiol.1992.sp019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eivindvik K., Sjogren C. E. Physicochemical properties of iodixanol. Acta Radiol Suppl. 1995;399:32–38. doi: 10.1177/0284185195036s39904. [DOI] [PubMed] [Google Scholar]
- Gomi N. Vasoconstriction by angiographic contrast media in isolated canine arteries. Br J Radiol. 1992 Nov;65(779):961–967. doi: 10.1259/0007-1285-65-779-961. [DOI] [PubMed] [Google Scholar]
- Jansen I., Golman K., Edvinsson L. Mechanisms of action of contrast media on cranial vessels. Comparison of diatrizoate, ioxaglate, iohexol, mannitol, and NaCl on rabbit basilar and ear arteries. Invest Radiol. 1987 Oct;22(10):814–821. doi: 10.1097/00004424-198710000-00008. [DOI] [PubMed] [Google Scholar]
- Kim D., Cragoe E. J., Jr, Smith T. W. Relations among sodium pump inhibition, Na-Ca and Na-H exchange activities, and Ca-H interaction in cultured chick heart cells. Circ Res. 1987 Feb;60(2):185–193. doi: 10.1161/01.res.60.2.185. [DOI] [PubMed] [Google Scholar]
- Lardani H., Rinaldi G., Cingolani H. Effects of angiographic contrast medium on isolated canine coronary arteries. Am J Cardiol. 1982 Oct;50(4):869–873. doi: 10.1016/0002-9149(82)91247-4. [DOI] [PubMed] [Google Scholar]
- Levitsky D. O., Benevolensky D. S. Effects of changing Ca2+-to-H+ ratio on Ca2+ uptake by cardiac sarcoplasmic reticulum. Am J Physiol. 1986 Mar;250(3 Pt 2):H360–H365. doi: 10.1152/ajpheart.1986.250.3.H360. [DOI] [PubMed] [Google Scholar]
- Low A. M., Loke J. C., Kwan C. Y., Daniel E. E. Sensitivity to protein kinase C inhibitors of nicardipine-insensitive component of high K+ contracture in rat and guinea-pig aorta. Br J Pharmacol. 1994 Jun;112(2):604–610. doi: 10.1111/j.1476-5381.1994.tb13117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. D., Etter E. F., Philipson K. D., Carrington W. A., Fogarty K. E., Lifshitz L. M., Fay F. S. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993 Oct 14;365(6447):657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Karaki H., Urakawa N. Possible role of Na-Ca exchange mechanism in the contractions induced in guinea-pig aorta by potassium free solution and ouabain. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;304(3):203–209. doi: 10.1007/BF00507959. [DOI] [PubMed] [Google Scholar]
- Pugh N. D., Griffith T. M., Karlsson J. O. Effects of iodinated contrast media on peripheral blood flow. Acta Radiol Suppl. 1995;399:155–163. doi: 10.1177/0284185195036s39918. [DOI] [PubMed] [Google Scholar]
- Pugh N. D., Hutcheson I. R., Edwards D. H., Nossen J. O., Karlsson J. O., Griffith T. M. Angiographic contrast media relax isolated rabbit aorta through an endothelium-independent mechanism that may not depend on the presence of the iodine atom. Br J Radiol. 1995 Jan;68(805):23–26. doi: 10.1259/0007-1285-68-805-23. [DOI] [PubMed] [Google Scholar]
- Pugh N. D., Sissons G. R., Ruttley M. S., Berg K. J., Nossen J. O., Eide H. Iodixanol in femoral arteriography (phase III): a comparative double-blind parallel trial between iodixanol and iopromide. Clin Radiol. 1993 Feb;47(2):96–99. doi: 10.1016/s0009-9260(05)81180-8. [DOI] [PubMed] [Google Scholar]
- Pugh N. D., Sissons G. R., Ruttley M. The effect of iodixanol, a new isotonic contrast agent, on femoral blood flow in man. Clin Radiol. 1992 Apr;45(4):243–245. doi: 10.1016/s0009-9260(05)80006-6. [DOI] [PubMed] [Google Scholar]
- Rocha G., Bucher B., Tschöpl M., Stoclet J. C. Hyperosmolarity enhances smooth muscle contractile responses to phenylephrine and partially impairs nitric oxide production in the rat tail artery. J Vasc Res. 1995 Jan-Feb;32(1):58–65. doi: 10.1159/000159078. [DOI] [PubMed] [Google Scholar]
- Sasaki N., Mitsuiye T., Wang Z., Noma A. Increase of the delayed rectifier K+ and Na(+)-K+ pump currents by hypotonic solutions in guinea pig cardiac myocytes. Circ Res. 1994 Nov;75(5):887–895. doi: 10.1161/01.res.75.5.887. [DOI] [PubMed] [Google Scholar]
- Sato K., Aoki K. Biphasic contraction induced by ouabain in human umbilical arteries. Eur J Pharmacol. 1988 Dec 13;158(3):299–302. doi: 10.1016/0014-2999(88)90084-2. [DOI] [PubMed] [Google Scholar]
- Sato K., Aoki K. Early and late contraction induced by ouabain in human umbilical arteries. Br J Pharmacol. 1991 Jun;103(2):1525–1529. doi: 10.1111/j.1476-5381.1991.tb09821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Lyu R. M., Smith L. Inhibition of sodium-calcium and sodium-proton exchangers by amiloride congeners in arterial muscle cells. Biochem Pharmacol. 1991 Feb 15;41(4):601–609. doi: 10.1016/0006-2952(91)90633-g. [DOI] [PubMed] [Google Scholar]
- Soleimani M., Singh G., Dominguez J. H., Howard R. L. Long-term high osmolality activates Na(+)-H+ exchange and protein kinase C in aortic smooth muscle cells. Circ Res. 1995 Apr;76(4):530–535. doi: 10.1161/01.res.76.4.530. [DOI] [PubMed] [Google Scholar]
- Toda N., Ayajiki K., Toda H., Hatano Y., Okamura T. Mechanism underlying mannitol-induced relaxation in isolated monkey cerebral arteries. Am J Physiol. 1992 Mar;262(3 Pt 2):H897–H902. doi: 10.1152/ajpheart.1992.262.3.H897. [DOI] [PubMed] [Google Scholar]
- Verow P., Nossen J. O., Sheppick A., Kjaersgaard P. A comparison of iodixanol with iopamidol in aorto-femoral angiography. Br J Radiol. 1995 Sep;68(813):973–978. doi: 10.1259/0007-1285-68-813-973. [DOI] [PubMed] [Google Scholar]
- Whalley D. W., Hemsworth P. D., Rasmussen H. H. Sodium-hydrogen exchange in guinea-pig ventricular muscle during exposure to hyperosmolar solutions. J Physiol. 1991 Dec;444:193–212. doi: 10.1113/jphysiol.1991.sp018873. [DOI] [PMC free article] [PubMed] [Google Scholar]


