Abstract
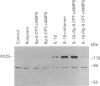
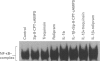
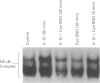
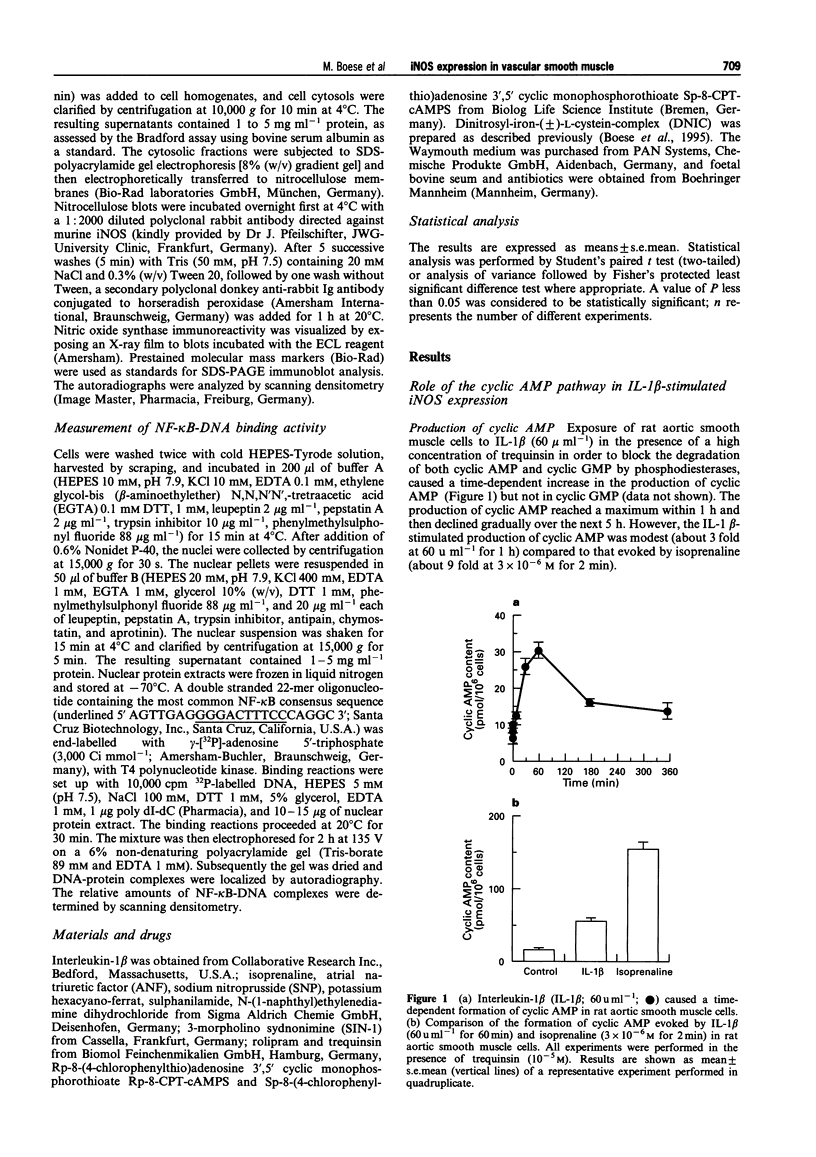
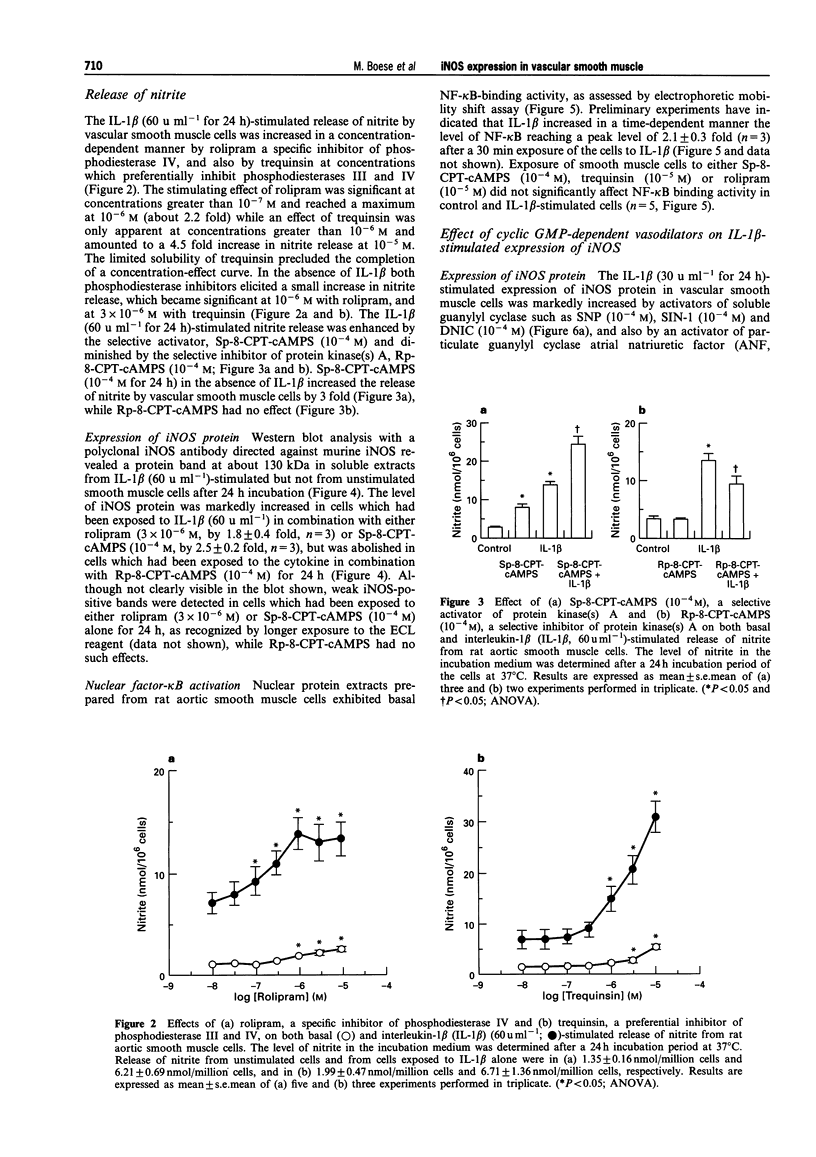
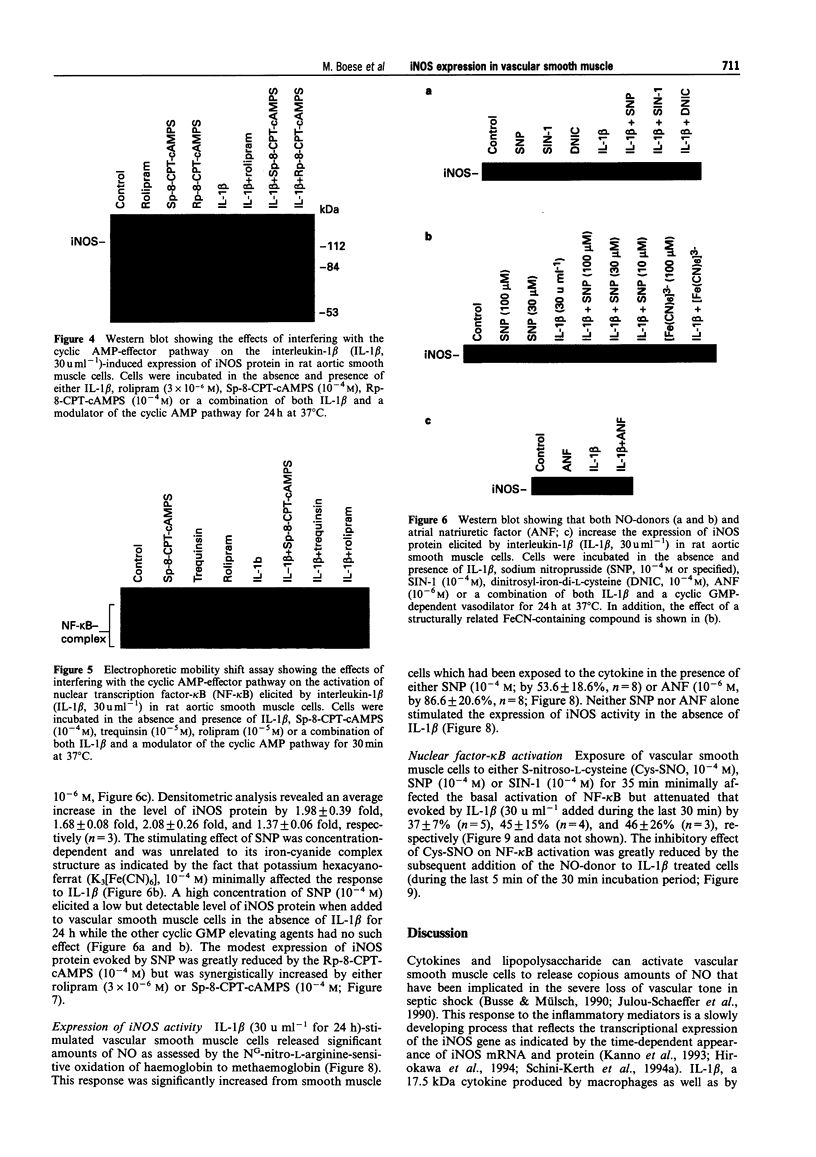
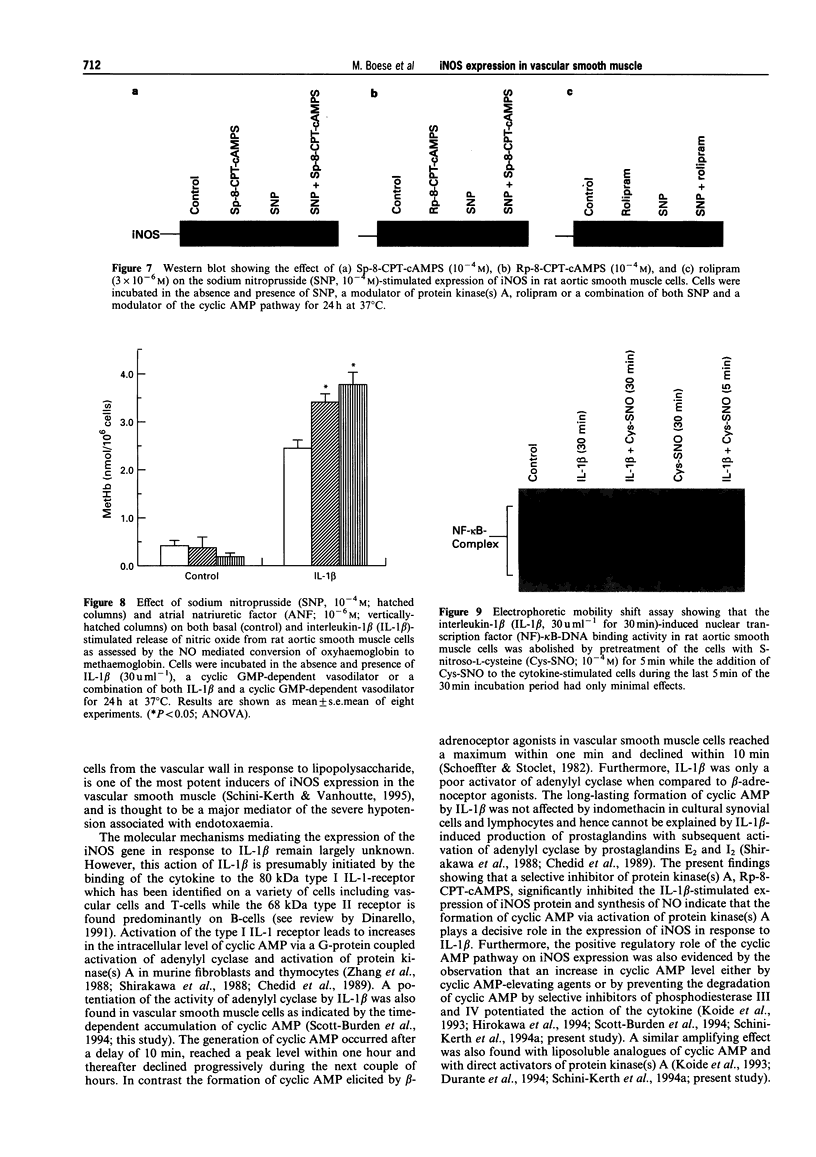
1. In the present study we examined whether interleukin-1 beta (IL-1 beta) increases the activity of adenylyl cyclase in vascular smooth muscle cells and determined its role in the cytokine-induced expression of the inducible nitric oxide synthase (iNOS) and activation of nuclear transcription factor-kappa B (NF-kappa B). In addition the interaction between cyclic AMP- and cyclic GMP-elevating agonists on the IL-1 beta-stimulated expression of iNOS was examined. 2. Exposure of vascular smooth muscle cells to IL-1 beta stimulated the formation of cyclic AMP but not of cyclic GMP. The intracellular level of cyclic AMP reached a maximum within 1 h and then gradually declined over the next 5 h. This IL-1 beta (60 u ml-1)-stimulated formation of cyclic AMP was modest (about 3 fold at 60 u ml-1 for 1 h) compared to that evoked by isoprenaline (about 9 fold at 3 x 10(-6) M for 2 min). 3. The IL-1 beta (60 u ml-1 for 24 h)-stimulated accumulation of nitrite, which was taken as an index of NO production, was concentration-dependently increased by preferential inhibitors of cyclic AMP-dependent phosphodiesterases (rolipram and trequinsin). This effect was reproduced by a specific activator of the cyclic AMP-dependent protein kinase(s) A, Sp-8-CPT-cAMPS (10(-4) M) but was prevented by a specific inhibitor of cyclic AMP-dependent protein kinase(s) A, Rp-8-CPT-cAMPS (10(-4) M). These compounds alone [rolipram (10(-6) M), trequinsin (3 x 10(-6) M) and Sp-8-CPT-cAMPS (10(-4) M)] slightly but significantly increased the release of nitric oxide while Rp-8-CPT-cAMPS elicited no such effect. 4. Inducible NOS protein was expressed in IL-1 beta (30 u ml-1, 24 h)-stimulated smooth muscle cells as assessed by Western blot analysis. The level of iNOS protein was markedly increased in smooth muscle cells which had been exposed to IL-1 beta in combination with either rolipram (3 x 10(-6) M) or Sp-8-CPT-cAMPS (10(-4) M) but was reduced in those exposed to IL-1 beta and Rp-8-CPT-cAMPS (10(-4) M). A weak expression of iNOS protein was found in smooth muscle cells which had been exposed to either Sp-8-CPT-cAMPS or rolipram alone for 24 h while Rp-8-CPT-cAMPS elicited no such effect. 5. Exposure of smooth muscle cells to IL-1 beta (30 u ml-1) for 30 min increased the level of NF-kappa B-DNA complexes in nuclear extracts as detected by electrophoretic mobility shift assay. Similar levels of NF-kappa B-DNA complexes were found in cells which had been exposed to IL-1 beta in combination with either Sp-8-CPT-cAMPS (10(-4) M), trequinsin (10(-6) M) or rolipram (10(-6) M). None of the modulators alone affected the basal level of NF-kappa B binding activity. 6. NO-donors [sodium nitroprusside (SNP) 10(-4) M; dinitrosyl-iron-di-L-cysteine-complex (DNIC), 10(-4) M; 3-morpholino-sydnonimine (SIN-1), 10(-4) M] and atrial natriuretic factor (10(-6) M) significantly increased the IL-1 beta (30 or 60 u ml-1, 24 h)-stimulated expression of iNOS protein and activity as assessed indirectly by the conversion of oxyhaemoglobin to methaemoglobin. In the absence of IL-1 beta, SNP (10(-4) M, 24 h) but not the other cyclic GMP-dependent vasodilators caused a modest expression of iNOS protein. No such effect was found in smooth muscle cells exposed to SNP in combination with Rp-8-CPT-cAMPS (10(-4) M) while an increased level of iNOS protein was found in those exposed to SNP in combination with either Sp-8-CPT-cAMPS (10(-4) M) or rolipram (3 x 10(-6) M). 7. Exposure of vascular smooth muscle cells to either S-nitroso-L-cysteine (Cys-SNO, 10(-4) M), SNP (10(-4) M) or SIN-1 (10(-4) M) for 35 min affected minimally the basal activation of NF-kappa B but abolished that evoked by IL-1 beta (30 u ml-1 added during the last 30 min). However, addition of Cys-SNO following the stimulation with IL-1 beta (during the last 5 min of the 30 min exposure period) reduced the level of NF-kappa B-DNA complexes only slightly. 8. These data indicate that the cyclic AMP-dependent pathway plays a decisi
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assender J. W., Southgate K. M., Hallett M. B., Newby A. C. Inhibition of proliferation, but not of Ca2+ mobilization, by cyclic AMP and GMP in rabbit aortic smooth-muscle cells. Biochem J. 1992 Dec 1;288(Pt 2):527–532. doi: 10.1042/bj2880527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley D., Schwartz J. H., Brenner B. M. Interleukin 1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991 Feb;87(2):602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Boese M., Mordvintcev P. I., Vanin A. F., Busse R., Mülsch A. S-nitrosation of serum albumin by dinitrosyl-iron complex. J Biol Chem. 1995 Dec 8;270(49):29244–29249. doi: 10.1074/jbc.270.49.29244. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Chedid M., Shirakawa F., Naylor P., Mizel S. B. Signal transduction pathway for IL-1. Involvement of a pertussis toxin-sensitive GTP-binding protein in the activation of adenylate cyclase. J Immunol. 1989 Jun 15;142(12):4301–4306. [PubMed] [Google Scholar]
- Cornwell T. L., Arnold E., Boerth N. J., Lincoln T. M. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1994 Nov;267(5 Pt 1):C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Soff G. A., Traynor A. E., Lincoln T. M. Regulation of the expression of cyclic GMP-dependent protein kinase by cell density in vascular smooth muscle cells. J Vasc Res. 1994 Nov-Dec;31(6):330–337. doi: 10.1159/000159061. [DOI] [PubMed] [Google Scholar]
- Durante W., Cheng K., Schafer A. I. Cyclic nucleotide regulation of interleukin-1 beta induced nitric oxide synthase expression in vascular smooth muscle cells. Thromb Res. 1994 Jul 1;75(1):63–71. doi: 10.1016/0049-3848(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Durante W., Schini V. B., Scott-Burden T., Junquero D. C., Kroll M. H., Vanhoutte P. M., Schafer A. I. Platelet inhibition by an L-arginine-derived substance released by IL-1 beta-treated vascular smooth muscle cells. Am J Physiol. 1991 Dec;261(6 Pt 2):H2024–H2030. doi: 10.1152/ajpheart.1991.261.6.H2024. [DOI] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Julou-Schaeffer G., Parratt J. R., Stoclet J. C. Incubation with endotoxin activates the L-arginine pathway in vascular tissue. Biochem Biophys Res Commun. 1990 Sep 14;171(2):562–568. doi: 10.1016/0006-291x(90)91183-s. [DOI] [PubMed] [Google Scholar]
- Forte L. R., Thorne P. K., Eber S. L., Krause W. J., Freeman R. H., Francis S. H., Corbin J. D. Stimulation of intestinal Cl- transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992 Sep;263(3 Pt 1):C607–C615. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Gordon D., Mohai L. G., Schwartz S. M. Induction of polyploidy in cultures of neonatal rat aortic smooth muscle cells. Circ Res. 1986 Dec;59(6):633–644. doi: 10.1161/01.res.59.6.633. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hirokawa K., O'Shaughnessy K., Moore K., Ramrakha P., Wilkins M. R. Induction of nitric oxide synthase in cultured vascular smooth muscle cells: the role of cyclic AMP. Br J Pharmacol. 1994 Jun;112(2):396–402. doi: 10.1111/j.1476-5381.1994.tb13085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Hirata Y., Kanno K., Marumo F. Induction of nitric oxide synthase by cyclic AMP in rat vascular smooth muscle cells. J Clin Invest. 1994 Feb;93(2):543–549. doi: 10.1172/JCI117005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Fukuo K., Nakahashi T., Hata S., Morimoto S., Ogihara T. cGMP upregulates nitric oxide synthase expression in vascular smooth muscle cells. Hypertension. 1995 Apr;25(4 Pt 2):711–714. doi: 10.1161/01.hyp.25.4.711. [DOI] [PubMed] [Google Scholar]
- Joly G. A., Schini V. B., Vanhoutte P. M. Balloon injury and interleukin-1 beta induce nitric oxide synthase activity in rat carotid arteries. Circ Res. 1992 Aug;71(2):331–338. doi: 10.1161/01.res.71.2.331. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kanno K., Hirata Y., Imai T., Marumo F. Induction of nitric oxide synthase gene by interleukin in vascular smooth muscle cells. Hypertension. 1993 Jul;22(1):34–39. doi: 10.1161/01.hyp.22.1.34. [DOI] [PubMed] [Google Scholar]
- Koide M., Kawahara Y., Nakayama I., Tsuda T., Yokoyama M. Cyclic AMP-elevating agents induce an inducible type of nitric oxide synthase in cultured vascular smooth muscle cells. Synergism with the induction elicited by inflammatory cytokines. J Biol Chem. 1993 Nov 25;268(33):24959–24966. [PubMed] [Google Scholar]
- Kunz D., Mühl H., Walker G., Pfeilschifter J. Two distinct signaling pathways trigger the expression of inducible nitric oxide synthase in rat renal mesangial cells. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5387–5391. doi: 10.1073/pnas.91.12.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. I., Weksler B. B., Jaffe E. A. The interaction of sodium nitroprusside with human endothelial cells and platelets: nitroprusside and prostacyclin synergistically inhibit platelet function. Circulation. 1982 Dec;66(6):1299–1307. doi: 10.1161/01.cir.66.6.1299. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993 Feb 1;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Link E., Kerr L. D., Schreck R., Zabel U., Verma I., Baeuerle P. A. Purified I kappa B-beta is inactivated upon dephosphorylation. J Biol Chem. 1992 Jan 5;267(1):239–246. [PubMed] [Google Scholar]
- Marumo T., Nakaki T., Hishikawa K., Hirahashi J., Suzuki H., Kato R., Saruta T. Natriuretic peptide-augmented induction of nitric oxide synthase through cyclic guanosine 3',5'-monophosphate elevation in vascular smooth muscle cells. Endocrinology. 1995 May;136(5):2135–2142. doi: 10.1210/endo.136.5.7536663. [DOI] [PubMed] [Google Scholar]
- Maurice D. H., Crankshaw D., Haslam R. J. Synergistic actions of nitrovasodilators and isoprenaline on rat aortic smooth muscle. Eur J Pharmacol. 1991 Jan 10;192(2):235–242. doi: 10.1016/0014-2999(91)90048-u. [DOI] [PubMed] [Google Scholar]
- Metz R., Ziff E. cAMP stimulates the C/EBP-related transcription factor rNFIL-6 to trans-locate to the nucleus and induce c-fos transcription. Genes Dev. 1991 Oct;5(10):1754–1766. doi: 10.1101/gad.5.10.1754. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Schray-Utz B., Mordvintcev P. I., Hauschildt S., Busse R. Diethyldithiocarbamate inhibits induction of macrophage NO synthase. FEBS Lett. 1993 Apr 26;321(2-3):215–218. doi: 10.1016/0014-5793(93)80111-7. [DOI] [PubMed] [Google Scholar]
- Peng H. B., Libby P., Liao J. K. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995 Jun 9;270(23):14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- Schini-Kerth V. B., Fisslthaler B., Busse R. CGRP enhances induction of NO synthase in vascular smooth muscle cells via a cAMP-dependent mechanism. Am J Physiol. 1994 Dec;267(6 Pt 2):H2483–H2490. doi: 10.1152/ajpheart.1994.267.6.H2483. [DOI] [PubMed] [Google Scholar]
- Schini-Kerth V. B., Vanhoutte P. M. Nitric oxide synthases in vascular cells. Exp Physiol. 1995 Nov;80(6):885–905. doi: 10.1113/expphysiol.1995.sp003904. [DOI] [PubMed] [Google Scholar]
- Schini-Kerth V., Bara A., Mülsch A., Busse R. Pyrrolidine dithiocarbamate selectively prevents the expression of the inducible nitric oxide synthase in the rat aorta. Eur J Pharmacol. 1994 Nov 14;265(1-2):83–87. doi: 10.1016/0014-2999(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Junquero D. C., Scott-Burden T., Vanhoutte P. M. Interleukin-1 beta induces the production of an L-arginine-derived relaxing factor from cultured smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1991 Apr 15;176(1):114–121. doi: 10.1016/0006-291x(91)90897-g. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Stoclet J. C. Age-related decrease of in vitro isoproterenol-induced cyclic AMP accumulation in rat aorta. Eur J Pharmacol. 1982 Jan 22;77(2-3):183–186. doi: 10.1016/0014-2999(82)90017-6. [DOI] [PubMed] [Google Scholar]
- Schuller F., Fleming I., Stoclet J. C., Gray G. A. Effect of endotoxin on circulating cyclic GMP in the rat. Eur J Pharmacol. 1992 Feb 25;212(1):93–96. doi: 10.1016/0014-2999(92)90077-h. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Elizondo E., Ge T., Boulanger C. M., Vanhoutte P. M. Simultaneous activation of adenylyl cyclase and protein kinase C induces production of nitric oxide by vascular smooth muscle cells. Mol Pharmacol. 1994 Aug;46(2):274–282. [PubMed] [Google Scholar]
- Scott-Burden T., Schini V. B., Elizondo E., Junquero D. C., Vanhoutte P. M. Platelet-derived growth factor suppresses and fibroblast growth factor enhances cytokine-induced production of nitric oxide by cultured smooth muscle cells. Effects on cell proliferation. Circ Res. 1992 Nov;71(5):1088–1100. doi: 10.1161/01.res.71.5.1088. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Aeberhard E. E., Wong V. Z., Griscavage J. M., Ignarro L. J. Pyrrolidine dithiocarbamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1301–1308. doi: 10.1006/bbrc.1993.1359. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Chedid M., Suttles J., Pollok B. A., Mizel S. B. Interleukin 1 and cyclic AMP induce kappa immunoglobulin light-chain expression via activation of an NF-kappa B-like DNA-binding protein. Mol Cell Biol. 1989 Mar;9(3):959–964. doi: 10.1128/mcb.9.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Yamashita U., Chedid M., Mizel S. B. Cyclic AMP--an intracellular second messenger for interleukin 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8201–8205. doi: 10.1073/pnas.85.21.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994 Feb 18;269(7):4705–4708. [PubMed] [Google Scholar]
- Xie Q. W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993 Jun 1;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q., Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994 Nov;56(5):576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- Zeiher A. M., Fisslthaler B., Schray-Utz B., Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995 Jun;76(6):980–986. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]
- Zhang Y. H., Lin J. X., Yip Y. K., Vilcek J. Enhancement of cAMP levels and of protein kinase activity by tumor necrosis factor and interleukin 1 in human fibroblasts: role in the induction of interleukin 6. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6802–6805. doi: 10.1073/pnas.85.18.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]