Abstract
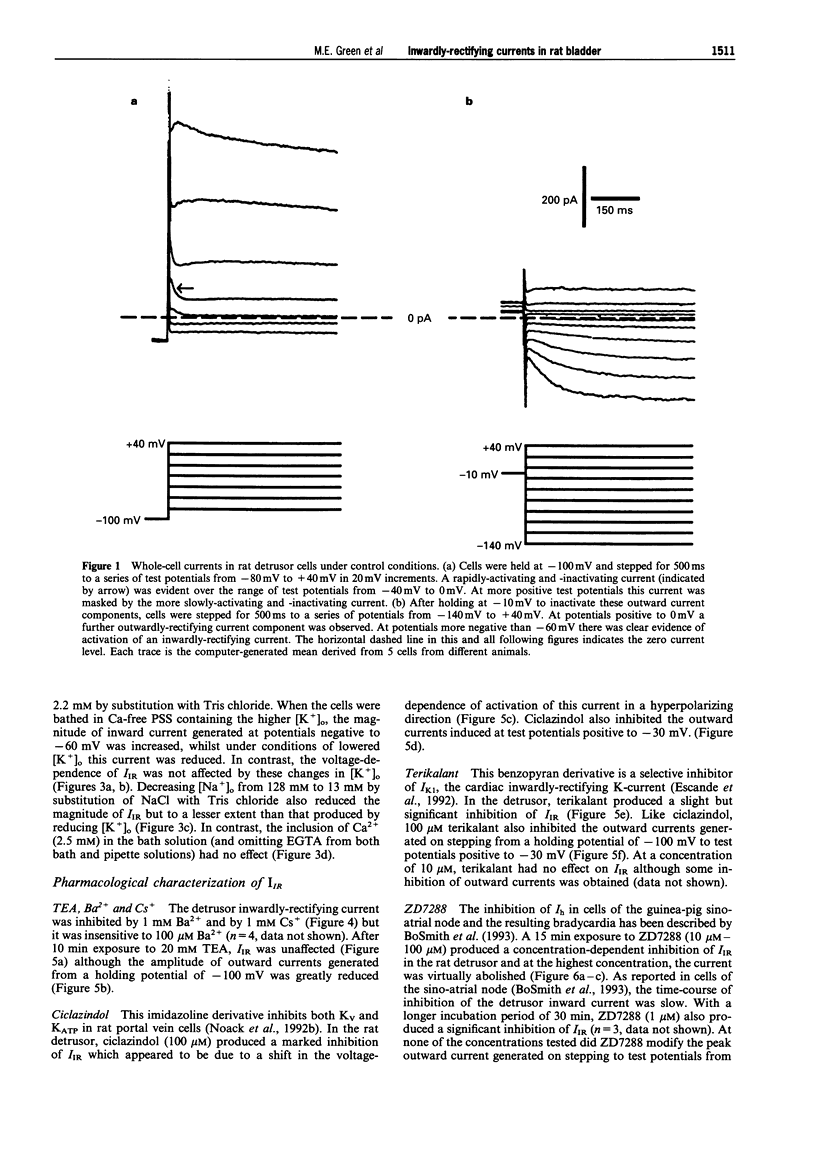
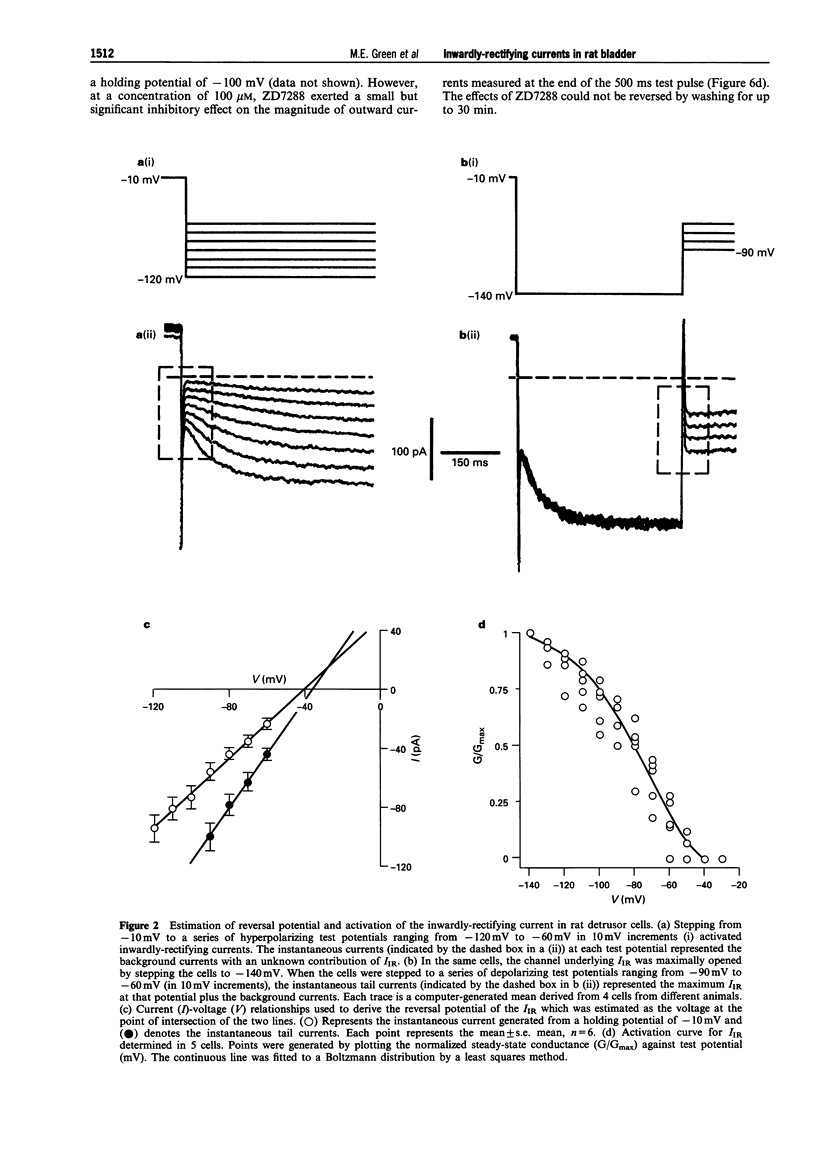
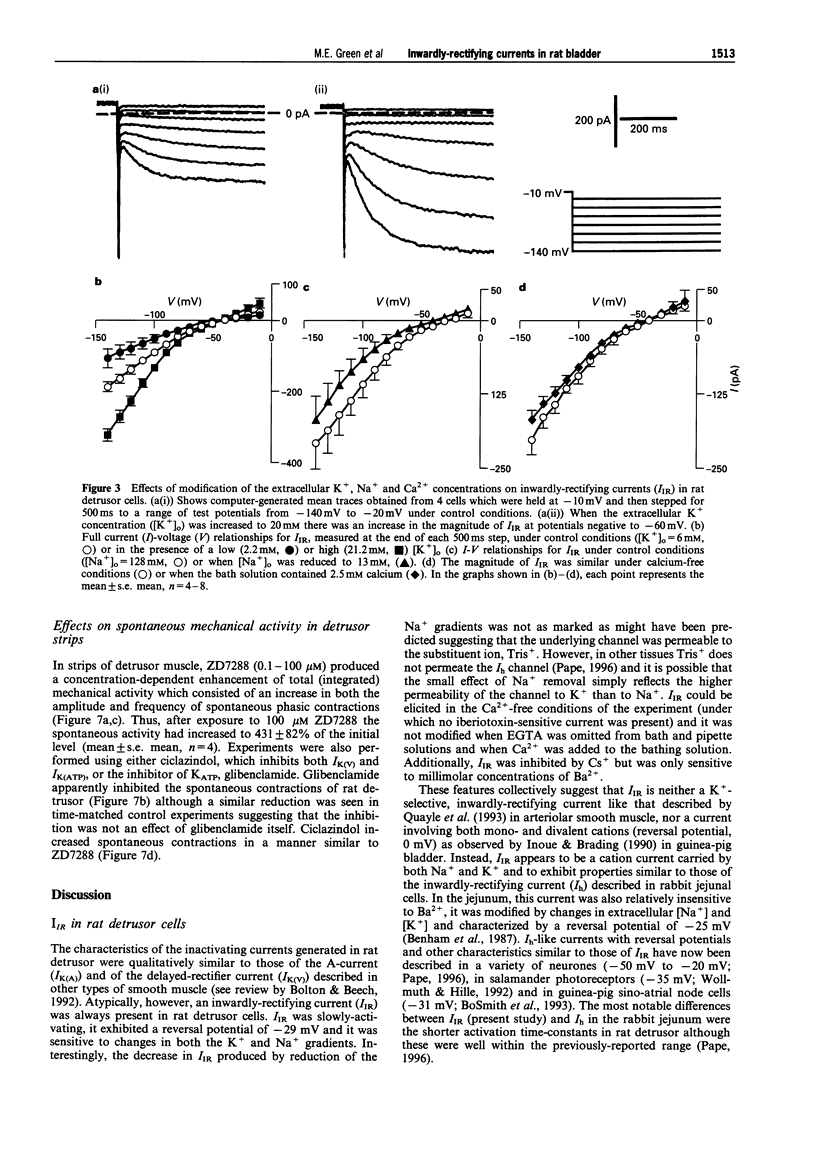
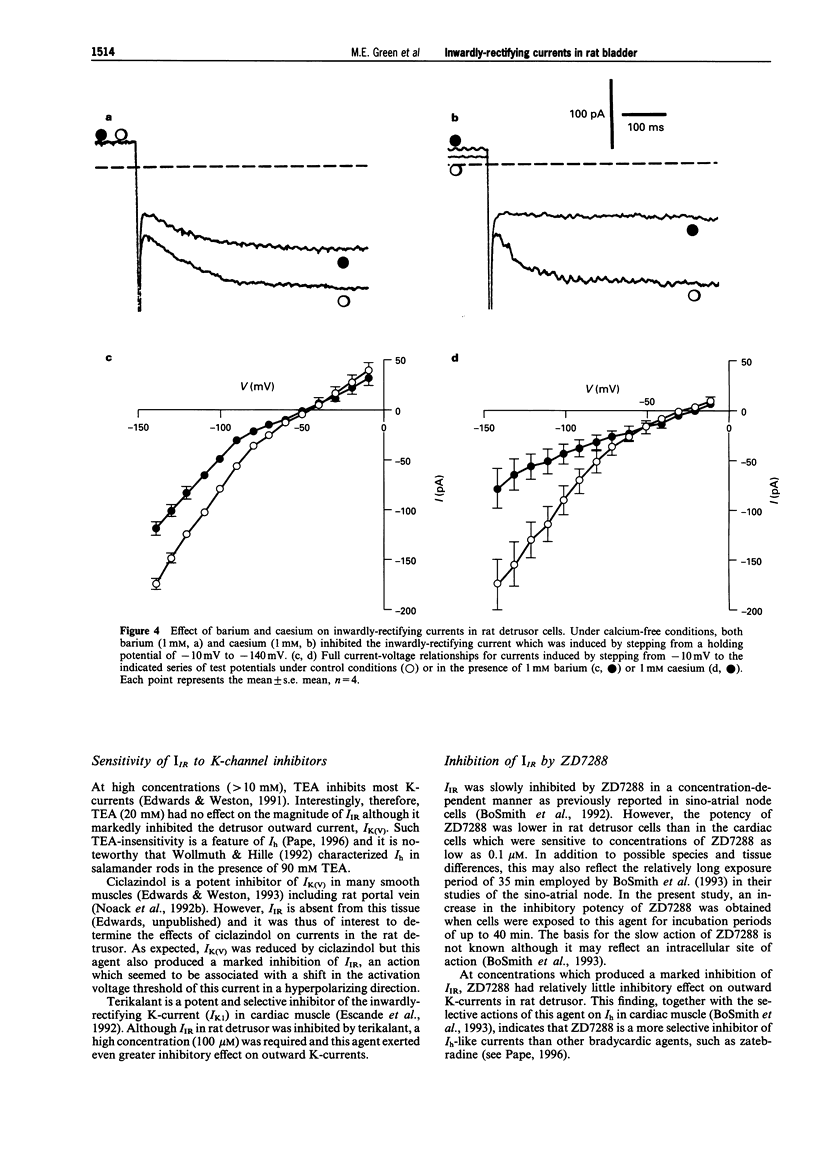
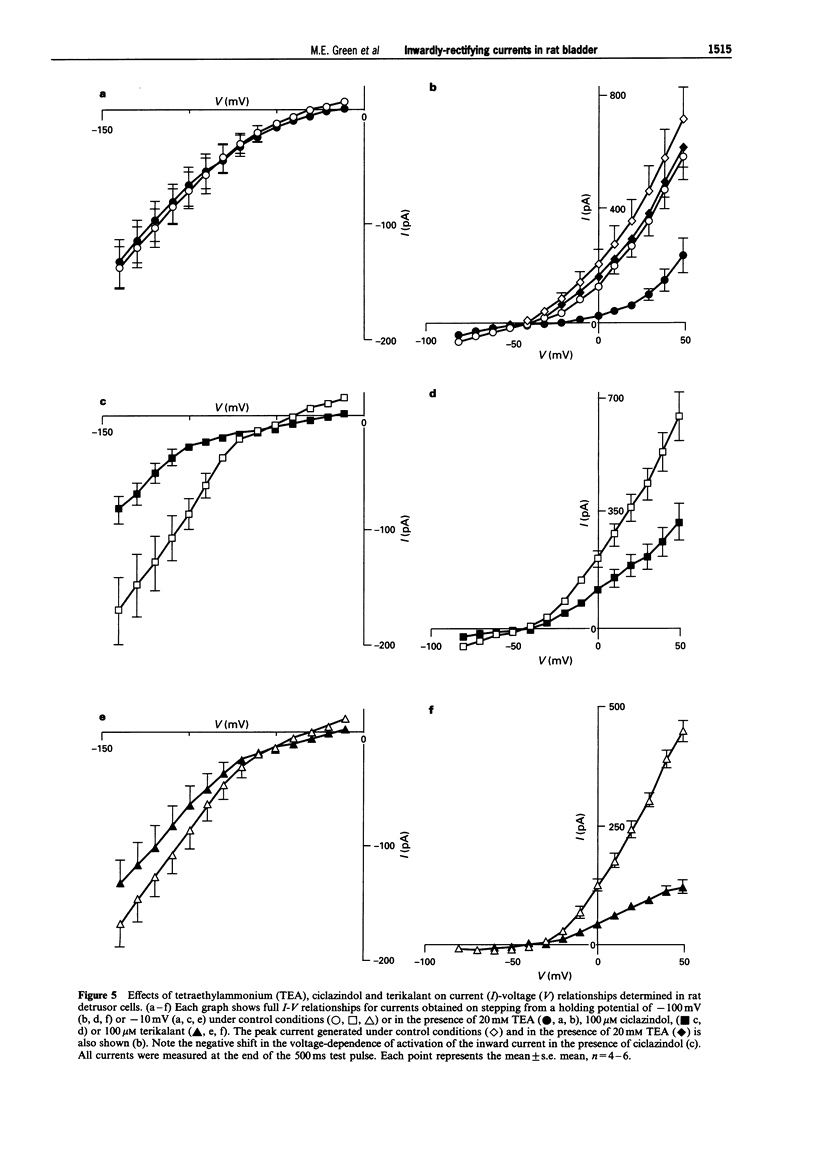
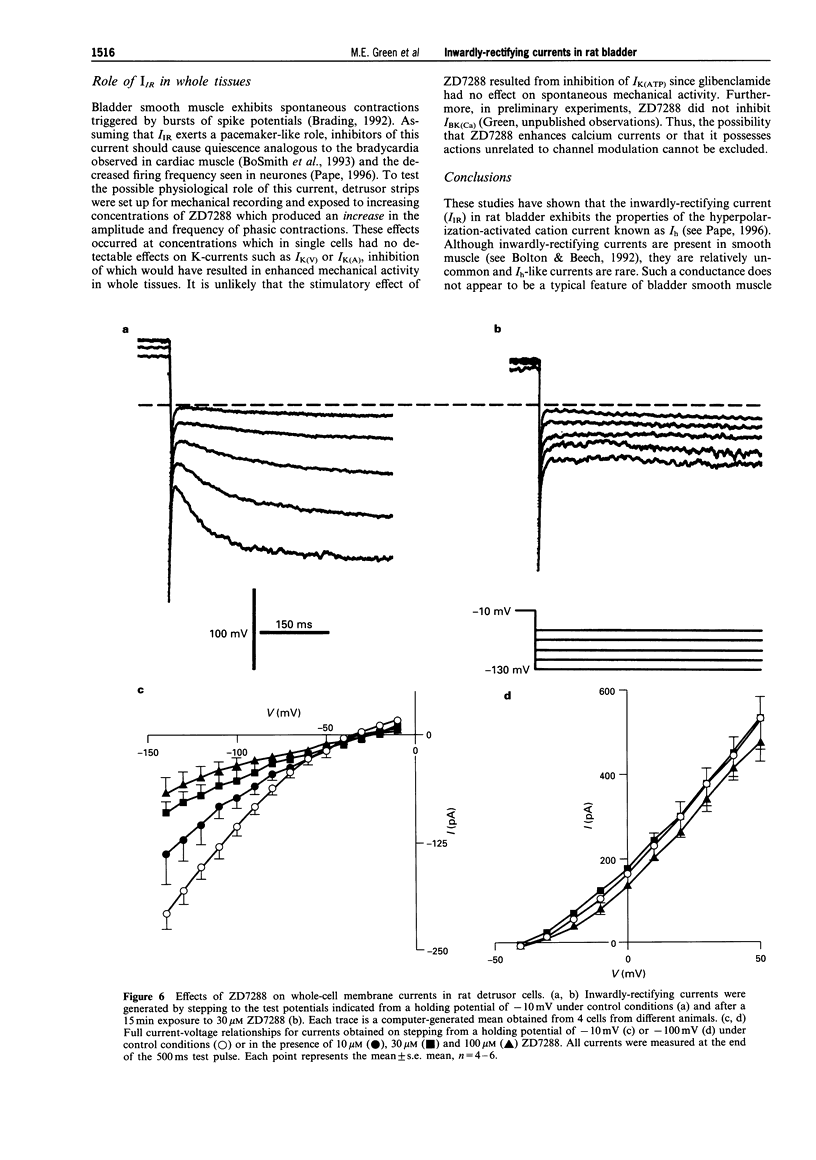
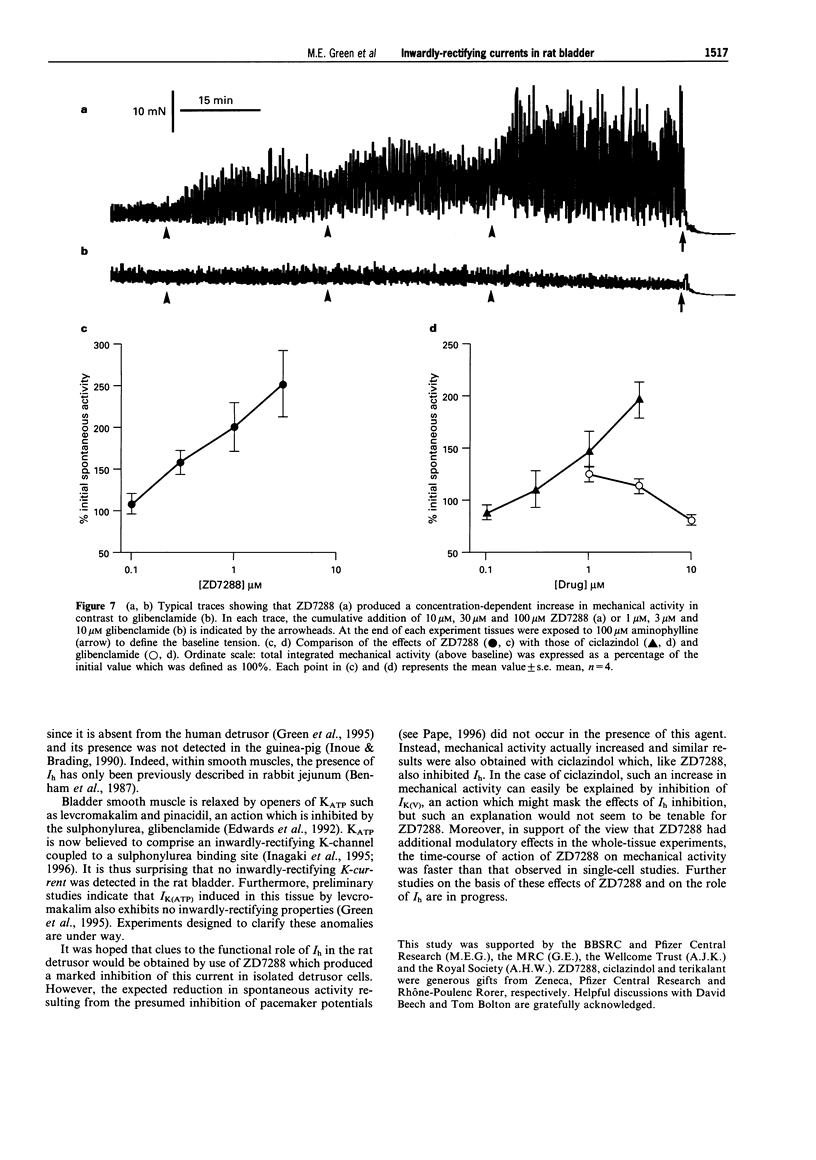
1. In freshly-isolated single cells of the rat bladder detrusor, outwardly-rectifying and inwardly-rectifying membrane currents were identified by the whole-cell voltage-clamp technique. 2. The inwardly-rectifying current (IIR) exhibited features of a cation current permeable to both K+ and Na+ but it was unaffected by changes in extracellular Ca2+. It had an activation threshold close to -60 mV and an estimated reversal potential of -29 mV. 3. IIR activated slowly with a voltage-sensitive time-constant of 69 ms at -140 mV and 209 ms at -100 mV but did not exhibit time-dependent inactivation. 4. IIR was unaffected by tetraethylammonium (up to 20 mM) but it was reduced by extracellular Ba2+ (1 mM) and by extracellular Cs+ (1 mM). 5. IIR was reduced by terikalant (100 microM) and markedly inhibited by ciclazindol (100 microM) although at these concentrations, both agents also reduced outward currents. 6. IIR was inhibited by ZD7288 (10-100 microM) in a concentration-dependent manner. At concentrations up to 30 microM, ZD7288 did not reduce the magnitude of outward currents but these were inhibited by 100 microM ZD7288. 7. In strips of bladder detrusor, spontaneous mechanical activity was increased by ZD7288 (0.3-100 microM) and by ciclazindol (0.3-100 microM) but was unaffected by glibenclamide (1-10 microM). 8. It is concluded that IIR closely resembles the hyperpolarization-activated current Ih, previously described in the smooth muscle of rabbit jejunum and in a variety of other cell types. This current may play an important role in modulating detrusor excitability but this could not be confirmed using the inhibitors ZD7288 and ciclazindol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B., Denbigh J. S., Lang R. J. Inward rectification in freshly isolated single smooth muscle cells of the rabbit jejunum. J Physiol. 1987 Feb;383:461–476. doi: 10.1113/jphysiol.1987.sp016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BoSmith R. E., Briggs I., Sturgess N. C. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol. 1993 Sep;110(1):343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Ion channels and control of contractile activity in urinary bladder smooth muscle. Jpn J Pharmacol. 1992;58 (Suppl 2):120P–127P. [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D. Inward rectification in submucosal arterioles of guinea-pig ileum. J Physiol. 1988 Oct;404:437–454. doi: 10.1113/jphysiol.1988.sp017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Hirst G. D., Silverberg G. D. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. J Physiol. 1988 Oct;404:455–466. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Henshaw M., Miller M., Weston A. H. Comparison of the effects of several potassium-channel openers on rat bladder and rat portal vein in vitro. Br J Pharmacol. 1991 Mar;102(3):679–686. doi: 10.1111/j.1476-5381.1991.tb12233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Escande D., Mestre M., Cavero I., Brugada J., Kirchhof C. RP 58866 and its active enantiomer RP 62719 (terikalant): blockers of the inward rectifier K+ current acting as pure class III antiarrhythmic agents. J Cardiovasc Pharmacol. 1992;20 (Suppl 2):S106–S113. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ibbotson T., Edwards G., Noack T., Weston A. H. Effects of P1060 and aprikalim on whole-cell currents in rat portal vein; inhibition by glibenclamide and phentolamine. Br J Pharmacol. 1993 Apr;108(4):991–998. doi: 10.1111/j.1476-5381.1993.tb13496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J. P., 4th, Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995 Nov 17;270(5239):1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J. P., Wang C. Z., Aguilar-Bryan L., Bryan J., Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996 May;16(5):1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack T., Deitmer P., Edwards G., Weston A. H. Characterization of potassium currents modulated by BRL 38227 in rat portal vein. Br J Pharmacol. 1992 Jul;106(3):717–726. doi: 10.1111/j.1476-5381.1992.tb14400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack T., Edwards G., Deitmer P., Greengrass P., Morita T., Andersson P. O., Criddle D., Wyllie M. G., Weston A. H. The involvement of potassium channels in the action of ciclazindol in rat portal vein. Br J Pharmacol. 1992 May;106(1):17–24. doi: 10.1111/j.1476-5381.1992.tb14286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H. C. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., McCarron J. G., Brayden J. E., Nelson M. T. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993 Nov;265(5 Pt 1):C1363–C1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- Wollmuth L. P., Hille B. Ionic selectivity of Ih channels of rod photoreceptors in tiger salamanders. J Gen Physiol. 1992 Nov;100(5):749–765. doi: 10.1085/jgp.100.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]


