Abstract
1. The liver has an important role in the detoxification of organic cations from the circulation. [3H]-1-methyl-4-phenylpyridinium ([3H]-MPP+), a low molecular weight organic cation, is efficiently taken up and accumulated by rat hepatocytes through mechanisms partially unknown. 2. The aim of the present work was to characterize further the uptake of MPP+ by rat isolated hepatocytes. The putative interactions of a wide range of drugs, including inhibitors/substrates of P-glycoprotein, were studied. 3. The uptake of MPP+ was investigated in rat freshly isolated hepatocytes (incubated in Krebs-Henseleit medium with 200 nM [3H]-MPP+ for 5 min) and in the rat liver in situ (perfused with Krebs-Henseleit/BSA medium with 200 nM [3H]-MPP+ for 30 min). [3H]-MPP+ accumulation in the cells and in tissue was determined by liquid scintillation counting. 4. Verapamil (100 microM), quinidine (100 microM), amiloride (1 mM), (+)-tubocurarine (100 microM), vecuronium (45 microM), bilirubin (200 microM), progesterone (200 microM), daunomycin (100 microM), vinblastine (100 microM), cyclosporin A (100 microM) and cimetidine (100 microM) had a significant inhibitory effect on the accumulation of [3H]-MPP+ in isolated hepatocytes. Tetraethylammonium (100 microM) had no effect. 5. In the rat perfused liver, both cyclosporin A (100 microM) and verapamil (100 microM) had much less marked inhibitory effects as compared to their effects on isolated hepatocytes (0% against 35% and 45% against 96% of inhibition, respectively). 6. Inhibition of alkaline phosphatase activity by increasing or decreasing the pH of the incubation medium or by the presence of vanadate (1 mM) or homoarginine (500 microM) led to a significant increase in the accumulation of [3H]-MPP+ in isolated hepatocytes. 7. It was concluded that, in addition to the type I organic cation hepatic transporter, [3H]-MPP+ is taken up by rat hepatocytes through P-glycoprotein, a canalicular transport system that usually excretes endobiotics and xenobiotics. We proposed that the reversal of transport through P-glycoprotein may be related to the loss of efficacy of alkaline in isolated hepatocytes.
Full text
PDF



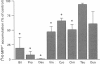
Images in this article
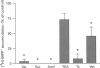
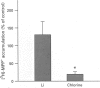
Selected References
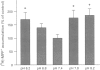
These references are in PubMed. This may not be the complete list of references from this article.
- Altenberg G. A., Young G., Horton J. K., Glass D., Belli J. A., Reuss L. Changes in intra- or extracellular pH do not mediate P-glycoprotein-dependent multidrug resistance. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9735–9738. doi: 10.1073/pnas.90.20.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S. E., Currier S. J., Alvarez M., Fojo A. T. Modulation of P-glycoprotein phosphorylation and drug transport by sodium butyrate. Biochemistry. 1992 Jul 21;31(28):6366–6372. doi: 10.1021/bi00143a002. [DOI] [PubMed] [Google Scholar]
- Becq F., Jensen T. J., Chang X. B., Savoia A., Rommens J. M., Tsui L. C., Buchwald M., Riordan J. R., Hanrahan J. W. Phosphatase inhibitors activate normal and defective CFTR chloride channels. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9160–9164. doi: 10.1073/pnas.91.19.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonet M. L., Llorca F. I., Cadenas E. Kinetic mechanism of Halobacterium halobium Mn(2+)-activated alkaline phosphatase. Biochem Mol Biol Int. 1994 Dec;34(6):1109–1120. [PubMed] [Google Scholar]
- Chambers T. C., McAvoy E. M., Jacobs J. W., Eilon G. Protein kinase C phosphorylates P-glycoprotein in multidrug resistant human KB carcinoma cells. J Biol Chem. 1990 May 5;265(13):7679–7686. [PubMed] [Google Scholar]
- Chan J. R., Stinson R. A. Dephosphorylation of phosphoproteins of human liver plasma membranes by endogenous and purified liver alkaline phosphatases. J Biol Chem. 1986 Jun 15;261(17):7635–7639. [PubMed] [Google Scholar]
- Di Monte D., Ekström G., Shinka T., Smith M. T., Trevor A. J., Castagnoli N., Jr Role of 1-methyl-4-phenylpyridinium ion formation and accumulation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity to isolated hepatocytes. Chem Biol Interact. 1987;62(2):105–116. doi: 10.1016/0009-2797(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Dutt A., Heath L. A., Nelson J. A. P-glycoprotein and organic cation secretion by the mammalian kidney. J Pharmacol Exp Ther. 1994 Jun;269(3):1254–1260. [PubMed] [Google Scholar]
- Eaton D. L., Klaassen C. D. Carrier-mediated transport of the organic cation procaine amide ethobromide by isolated rat liver parenchymal cells. J Pharmacol Exp Ther. 1978 Sep;206(3):595–606. [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gründemann D., Gorboulev V., Gambaryan S., Veyhl M., Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994 Dec 8;372(6506):549–552. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- Horio M., Chin K. V., Currier S. J., Goldenberg S., Williams C., Pastan I., Gottesman M. M., Handler J. Transepithelial transport of drugs by the multidrug transporter in cultured Madin-Darby canine kidney cell epithelia. J Biol Chem. 1989 Sep 5;264(25):14880–14884. [PubMed] [Google Scholar]
- Hsing S., Gatmaitan Z., Arias I. M. The function of Gp170, the multidrug-resistance gene product, in the brush border of rat intestinal mucosa. Gastroenterology. 1992 Mar;102(3):879–885. doi: 10.1016/0016-5085(92)90173-v. [DOI] [PubMed] [Google Scholar]
- Hu D. E., Fan T. P. Suppression of VEGF-induced angiogenesis by the protein tyrosine kinase inhibitor, lavendustin A. Br J Pharmacol. 1995 Jan;114(2):262–268. doi: 10.1111/j.1476-5381.1995.tb13221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Karlsson J., Kuo S. M., Ziemniak J., Artursson P. Transport of celiprolol across human intestinal epithelial (Caco-2) cells: mediation of secretion by multiple transporters including P-glycoprotein. Br J Pharmacol. 1993 Nov;110(3):1009–1016. doi: 10.1111/j.1476-5381.1993.tb13914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C. D., Watkins J. B., 3rd Mechanisms of bile formation, hepatic uptake, and biliary excretion. Pharmacol Rev. 1984 Mar;36(1):1–67. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin C. W., Fishman W. H. L-Homoarginine. An organ-specific, uncompetitive inhibitor of human liver and bone alkaline phosphohydrolases. J Biol Chem. 1972 May 25;247(10):3082–3087. [PubMed] [Google Scholar]
- Ma L. D., Marquardt D., Takemoto L., Center M. S. Analysis of P-glycoprotein phosphorylation in HL60 cells isolated for resistance to vincristine. J Biol Chem. 1991 Mar 25;266(9):5593–5599. [PubMed] [Google Scholar]
- Martel F., Martins M. J., Azevedo I. Inward transport of 3H-MPP+ in freshly isolated rat hepatocytes: evidence for interaction with catecholamines. Naunyn Schmiedebergs Arch Pharmacol. 1996 Aug-Sep;354(3):305–311. doi: 10.1007/BF00171061. [DOI] [PubMed] [Google Scholar]
- Martel F., Vetter T., Russ H., Gründemann D., Azevedo I., Koepsell H., Schömig E. Transport of small organic cations in the rat liver. The role of the organic cation transporter OCT1. Naunyn Schmiedebergs Arch Pharmacol. 1996 Aug-Sep;354(3):320–326. doi: 10.1007/BF00171063. [DOI] [PubMed] [Google Scholar]
- Mazzanti R., Fantappié O., Kamimoto Y., Gatmaitan Z., Gentilini P., Arias I. M. Bile acid inhibition of P-glycoprotein-mediated transport in multidrug-resistant cells and rat liver canalicular membrane vesicles. Hepatology. 1994 Jul;20(1 Pt 1):170–176. doi: 10.1016/0270-9139(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Meijer D. K., Mol W. E., Müller M., Kurz G. Carrier-mediated transport in the hepatic distribution and elimination of drugs, with special reference to the category of organic cations. J Pharmacokinet Biopharm. 1990 Feb;18(1):35–70. doi: 10.1007/BF01063621. [DOI] [PubMed] [Google Scholar]
- Mellado W., Horwitz S. B. Phosphorylation of the multidrug resistance associated glycoprotein. Biochemistry. 1987 Nov 3;26(22):6900–6904. doi: 10.1021/bi00396a005. [DOI] [PubMed] [Google Scholar]
- Mol W. E., Fokkema G. N., Weert B., Meijer D. K. Mechanisms for the hepatic uptake of organic cations. Studies with the muscle relaxant vecuronium in isolated rat hepatocytes. J Pharmacol Exp Ther. 1988 Jan;244(1):268–275. [PubMed] [Google Scholar]
- Moss D. W. Alkaline phosphatase isoenzymes. Clin Chem. 1982 Oct;28(10):2007–2016. [PubMed] [Google Scholar]
- Moss D. W. Perspectives in alkaline phosphatase research. Clin Chem. 1992 Dec;38(12):2486–2492. [PubMed] [Google Scholar]
- Oude Elferink R. P., Meijer D. K., Kuipers F., Jansen P. L., Groen A. K., Groothuis G. M. Hepatobiliary secretion of organic compounds; molecular mechanisms of membrane transport. Biochim Biophys Acta. 1995 Jul 17;1241(2):215–268. doi: 10.1016/0304-4157(95)00006-d. [DOI] [PubMed] [Google Scholar]
- Pan B. F., Dutt A., Nelson J. A. Enhanced transepithelial flux of cimetidine by Madin-Darby canine kidney cells overexpressing human P-glycoprotein. J Pharmacol Exp Ther. 1994 Jul;270(1):1–7. [PubMed] [Google Scholar]
- Rennick B. R. Renal tubule transport of organic cations. Am J Physiol. 1981 Feb;240(2):F83–F89. doi: 10.1152/ajprenal.1981.240.2.F83. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Karnovsky M. J. Ultrastructural localization of several phosphatases with cerium. J Histochem Cytochem. 1983 Oct;31(10):1197–1208. doi: 10.1177/31.10.6309949. [DOI] [PubMed] [Google Scholar]
- Sikic B. I., Fisher G. A., Lum B. L., Brophy N. A., Yahanda A. M., Adler K. M., Halsey J. Clinical reversal of multidrug resistance. Cancer Treat Res. 1994;73:149–165. doi: 10.1007/978-1-4615-2632-2_8. [DOI] [PubMed] [Google Scholar]
- Singh Y., Swanson E., Sokoloski E., Kutty R. K., Krishna G. MPTP and MPTP analogs induced cell death in cultured rat hepatocytes involving the formation of pyridinium metabolites. Toxicol Appl Pharmacol. 1988 Nov;96(2):347–359. doi: 10.1016/0041-008x(88)90093-2. [DOI] [PubMed] [Google Scholar]
- Smit J. J., Schinkel A. H., Oude Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., van Roon M. A. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993 Nov 5;75(3):451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Staats J., Marquardt D., Center M. S. Characterization of a membrane-associated protein kinase of multidrug-resistant HL60 cells which phosphorylates P-glycoprotein. J Biol Chem. 1990 Mar 5;265(7):4084–4090. [PubMed] [Google Scholar]
- Thalhammer T., Stapf V., Gajdzik L., Graf J. Bile canalicular cationic dye secretion as a model for P-glycoprotein mediated transport. Eur J Pharmacol. 1994 Apr 4;270(2-3):213–220. doi: 10.1016/0926-6917(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Vorbrodt A. W., Trowbridge R. S. Ultracytochemical characteristics of cultured goat brain microvascular endothelial cells [corrected]. J Histochem Cytochem. 1991 Nov;39(11):1555–1563. doi: 10.1177/39.11.1655877. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Miyauchi S., Sawada Y., Iga T., Hanano M., Inaba M., Sugiyama Y. Kinetic analysis of hepatobiliary transport of vincristine in perfused rat liver. Possible roles of P-glycoprotein in biliary excretion of vincristine. J Hepatol. 1992 Sep;16(1-2):77–88. doi: 10.1016/s0168-8278(05)80098-4. [DOI] [PubMed] [Google Scholar]
- Zacherl J., Hamilton G., Thalhammer T., Riegler M., Cosentini E. P., Ellinger A., Bischof G., Schweitzer M., Teleky B., Koperna T. Inhibition of P-glycoprotein-mediated vinblastine transport across HCT-8 intestinal carcinoma monolayers by verapamil, cyclosporine A and SDZ PSC 833 in dependence on extracellular pH. Cancer Chemother Pharmacol. 1994;34(2):125–132. doi: 10.1007/BF00685929. [DOI] [PubMed] [Google Scholar]