Abstract
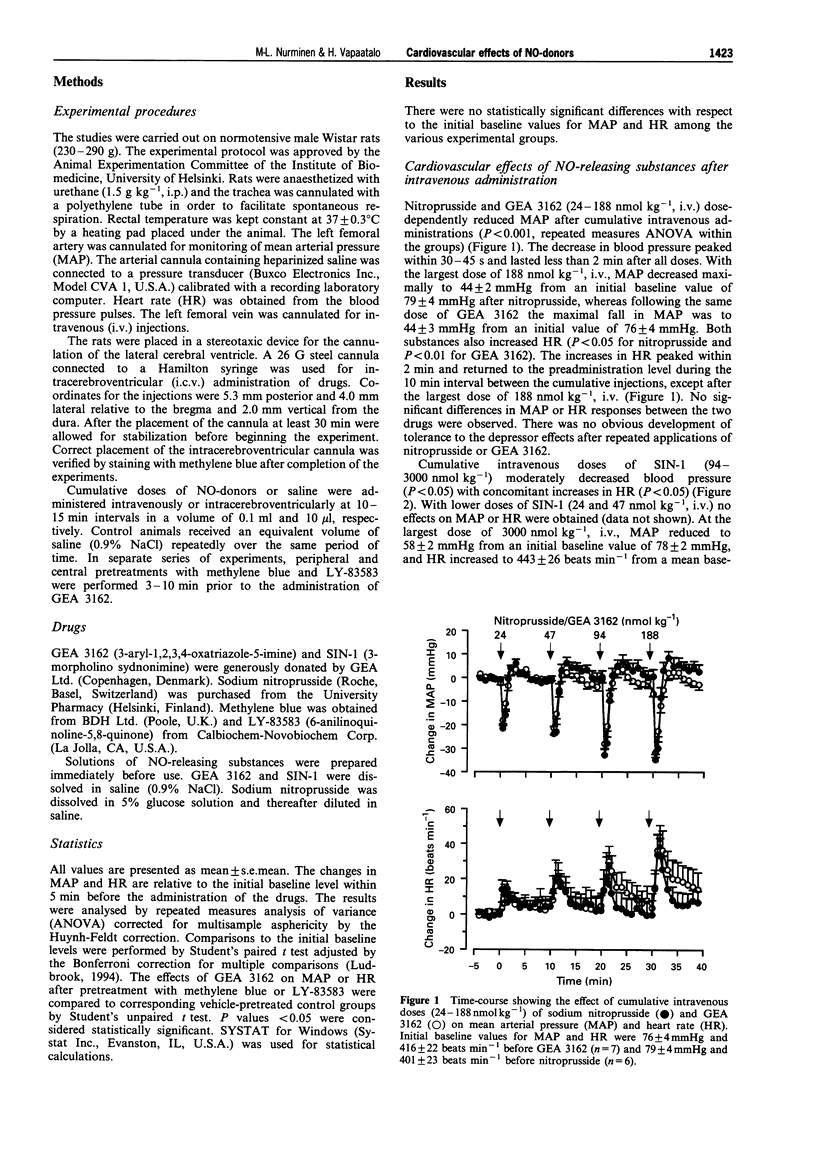
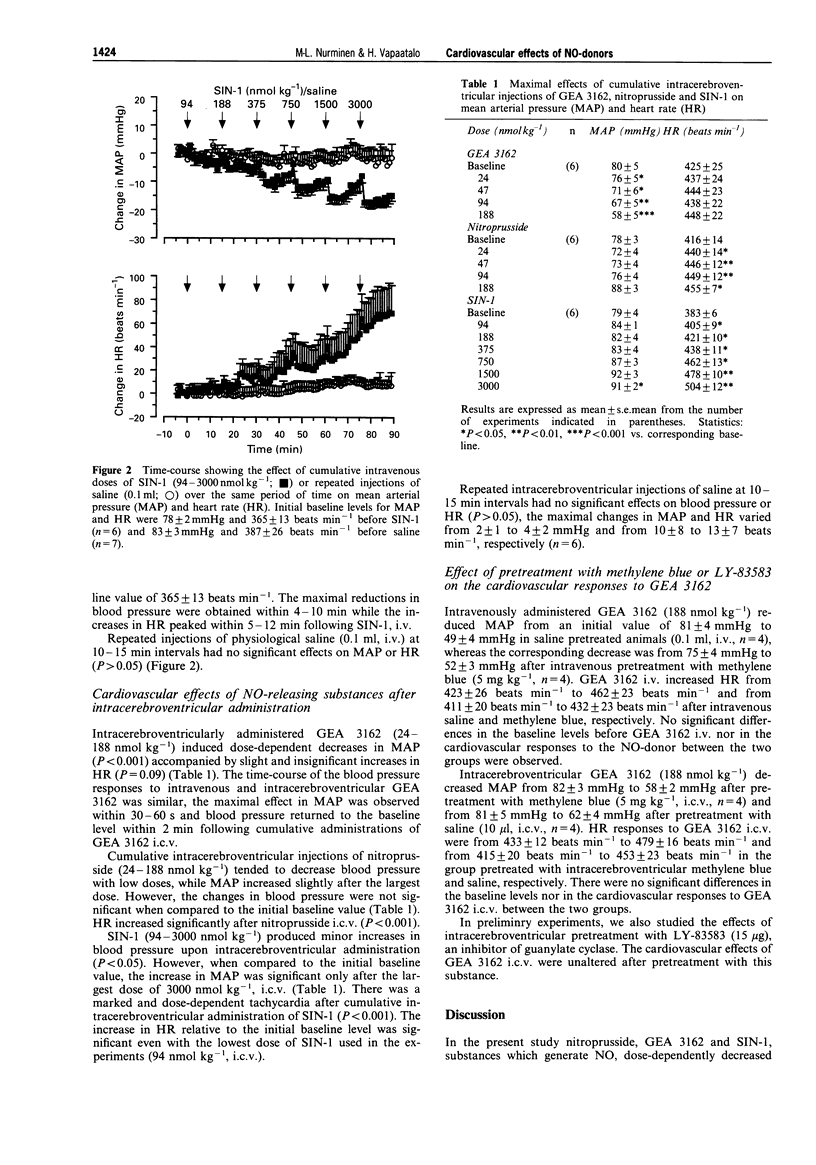
1. The effects of nitric oxide (NO) releasing substances, sodium nitroprusside, 3-morpholino sydnonimine (SIN-1) and a novel oxatriazole derivative, GEA 3162, on blood pressure and heart rate were studied after peripheral or central administration in anaesthetized normotensive Wistar rats. 2. Given as cumulative intravenous injections, both nitroprusside and GEA 3162 (24-188 nmol kg-1) induced short-lasting and dose-dependent decreases in mean arterial pressure, while SIN-1 decreased blood pressure only slightly even after larger doses (94-3000 nmol kg-1). Heart rate increased concomitantly with the hypotensive effect of the NO-releasing substances. 3. Cumulative intracerebroventricular administration of GEA 3162 (24-188 nmol kg-1) induced a dose-dependent hypotension with slight but insignificant increases in heart rate. In contrast, intracerebroventricular nitroprusside induced little change in blood pressure, while a large dose of SIN-1 (3000 nmol kg-1, i.c.v.) slightly increased mean arterial pressure. However, intracerebroventricular nitroprusside and SIN-1 increased heart rate at doses that did not significantly affect blood pressure. 4. To determine whether the cardiovascular effects of GEA 3162 were attributable to an elevation of cyclic GMP levels, pretreatments with methylene blue, a putative guanylate cyclase inhibitor, were performed. This substance failed to attenuate the cardiovascular effects of peripherally or centrally administered GEA 3162, suggesting that the effects were independent of guanylate cyclase. 5. In conclusion, the centrally administered NO-donor, GEA 3162, induced a dose-dependent. hypotensive response without significant changes in heart rate. Furthermore, intracerebroventricular injections of nitroprusside and SIN-1 increased heart rate without affecting blood pressure. These results suggest that NO released by these drugs may affect central mechanisms involved in cardiovascular regulation independently of cyclic GMP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates J. N., Baker M. T., Guerra R., Jr, Harrison D. G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Cabrera C., Bohr D. The role of nitric oxide in the central control of blood pressure. Biochem Biophys Res Commun. 1995 Jan 5;206(1):77–81. doi: 10.1006/bbrc.1995.1011. [DOI] [PubMed] [Google Scholar]
- Calapai G., Squadrito F., Altavilla D., Zingarelli B., Campo G. M., Cilia M., Caputi A. P. Evidence that nitric oxide modulates drinking behaviour. Neuropharmacology. 1992 Aug;31(8):761–764. doi: 10.1016/0028-3908(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Corell T., Pedersen S. B., Lissau B., Moilanen E., Metsä-Ketelä T., Kankaanranta H., Vuorinen P., Vapaatalo H., Rydell E., Andersson R. Pharmacology of mesoionic oxatriazole derivatives in blood, cardiovascular and respiratory systems. Pol J Pharmacol. 1994 Nov-Dec;46(6):553–566. [PubMed] [Google Scholar]
- Feelisch M., Ostrowski J., Noack E. On the mechanism of NO release from sydnonimines. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S13–S22. [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Zembowicz A., Salvemini D., Taylor G. W., Vane J. R. Modulation of the pharmacological actions of nitrovasodilators by methylene blue and pyocyanin. Br J Pharmacol. 1992 Aug;106(4):838–845. doi: 10.1111/j.1476-5381.1992.tb14422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., McArthur C., Grady C., Ruderman N. B. Stimulation of vascular Na(+)-K(+)-ATPase activity by nitric oxide: a cGMP-independent effect. Am J Physiol. 1994 May;266(5 Pt 2):H2146–H2151. doi: 10.1152/ajpheart.1994.266.5.H2146. [DOI] [PubMed] [Google Scholar]
- Hegde L. G., Shukla R., Dikshit M., Srimal R. C. Study on the involvement of the L-arginine/nitric oxide pathway in the central cardiovascular regulation in the chloralose-anaesthetized cat. Arch Int Pharmacodyn Ther. 1994 Sep-Oct;328(2):155–164. [PubMed] [Google Scholar]
- Horn T., Smith P. M., McLaughlin B. E., Bauce L., Marks G. S., Pittman Q. J., Ferguson A. V. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol. 1994 Jan;266(1 Pt 2):R306–R313. doi: 10.1152/ajpregu.1994.266.1.R306. [DOI] [PubMed] [Google Scholar]
- Kankaanranta H., Rydell E., Petersson A. S., Holm P., Moilanen E., Corell T., Karup G., Vuorinen P., Pedersen S. B., Wennmalm A. Nitric oxide-donating properties of mesoionic 3-aryl substituted oxatriazole-5-imine derivatives. Br J Pharmacol. 1996 Feb;117(3):401–406. doi: 10.1111/j.1476-5381.1996.tb15204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapás L., Shibata M., Kimura M., Krueger J. M. Inhibition of nitric oxide synthesis suppresses sleep in rabbits. Am J Physiol. 1994 Jan;266(1 Pt 2):R151–R157. doi: 10.1152/ajpregu.1994.266.1.R151. [DOI] [PubMed] [Google Scholar]
- Karup G., Preikschat H., Wilhelmsen E. S., Pedersen S. B., Marcinkiewicz E., Cieślik K., Gryglewski R. J. Mesoionic oxatriazole derivatives--a new group of NO-donors. Pol J Pharmacol. 1994 Nov-Dec;46(6):541–552. [PubMed] [Google Scholar]
- Ling L., Karius D. R., Fiscus R. R., Speck D. F. Endogenous nitric oxide required for an integrative respiratory function in the cat brain. J Neurophysiol. 1992 Nov;68(5):1910–1912. doi: 10.1152/jn.1992.68.5.1910. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Ludbrook J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc Res. 1994 Mar;28(3):303–311. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- Ma S., Long J. P. Central noradrenergic activity and the cardiovascular effects of nitroglycerin and amyl nitrate. J Cardiovasc Pharmacol. 1992;20(5):826–836. [PubMed] [Google Scholar]
- Molina y Vedia L., McDonald B., Reep B., Brüne B., Di Silvio M., Billiar T. R., Lapetina E. G. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem. 1992 Dec 15;267(35):24929–24932. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore P. K., Oluyomi A. O., Babbedge R. C., Wallace P., Hart S. L. L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br J Pharmacol. 1991 Jan;102(1):198–202. doi: 10.1111/j.1476-5381.1991.tb12153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Busse R., Liebau S., Förstermann U. LY 83583 interferes with the release of endothelium-derived relaxing factor and inhibits soluble guanylate cyclase. J Pharmacol Exp Ther. 1988 Oct;247(1):283–288. [PubMed] [Google Scholar]
- Ota M., Crofton J. T., Festavan G. T., Share L. Evidence that nitric oxide can act centrally to stimulate vasopressin release. Neuroendocrinology. 1993 May;57(5):955–959. doi: 10.1159/000126459. [DOI] [PubMed] [Google Scholar]
- Shapoval L. N., Sagach V. F., Pobegailo L. S. Nitric oxide influences ventrolateral medullary mechanisms of vasomotor control in the cat. Neurosci Lett. 1991 Oct 28;132(1):47–50. doi: 10.1016/0304-3940(91)90430-2. [DOI] [PubMed] [Google Scholar]
- Shibuki K., Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991 Jan 24;349(6307):326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- Southam E., Garthwaite J. Comparative effects of some nitric oxide donors on cyclic GMP levels in rat cerebellar slices. Neurosci Lett. 1991 Sep 2;130(1):107–111. doi: 10.1016/0304-3940(91)90239-p. [DOI] [PubMed] [Google Scholar]
- Togashi H., Sakuma I., Yoshioka M., Kobayashi T., Yasuda H., Kitabatake A., Saito H., Gross S. S., Levi R. A central nervous system action of nitric oxide in blood pressure regulation. J Pharmacol Exp Ther. 1992 Jul;262(1):343–347. [PubMed] [Google Scholar]
- Vincent S. R., Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46(4):755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]


