Abstract
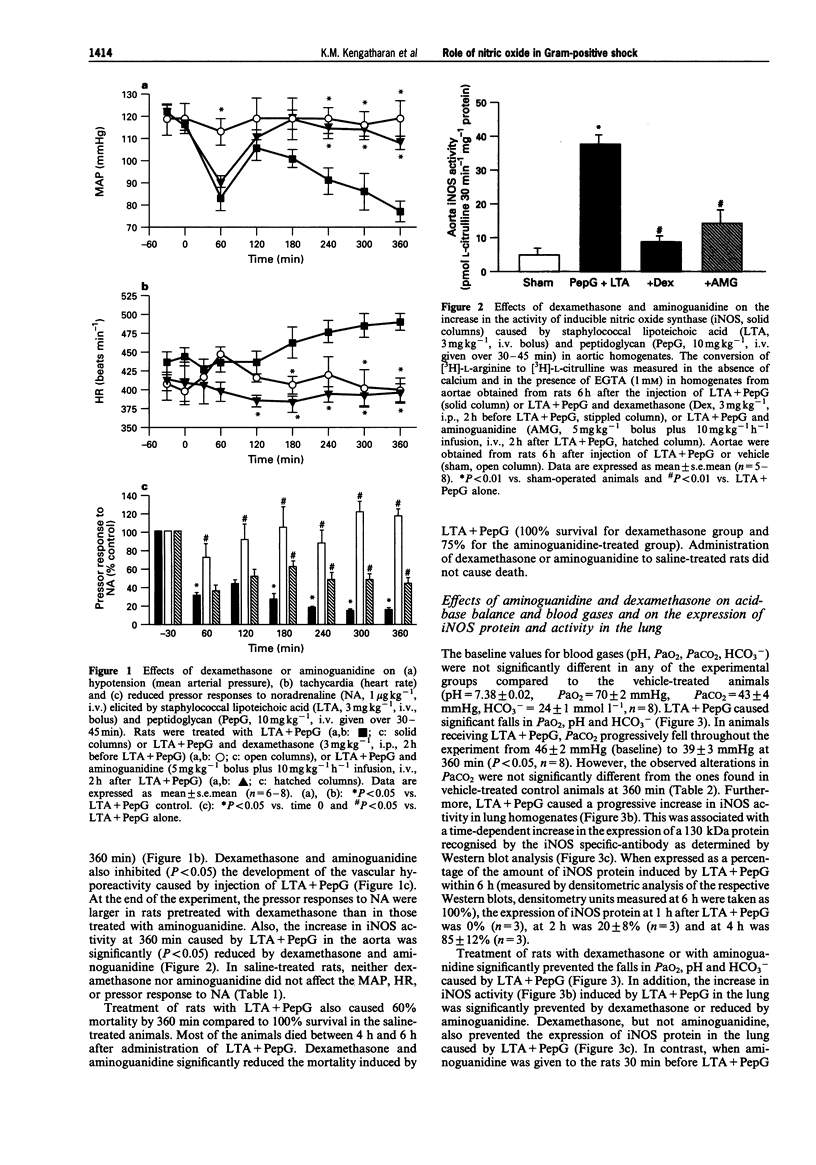
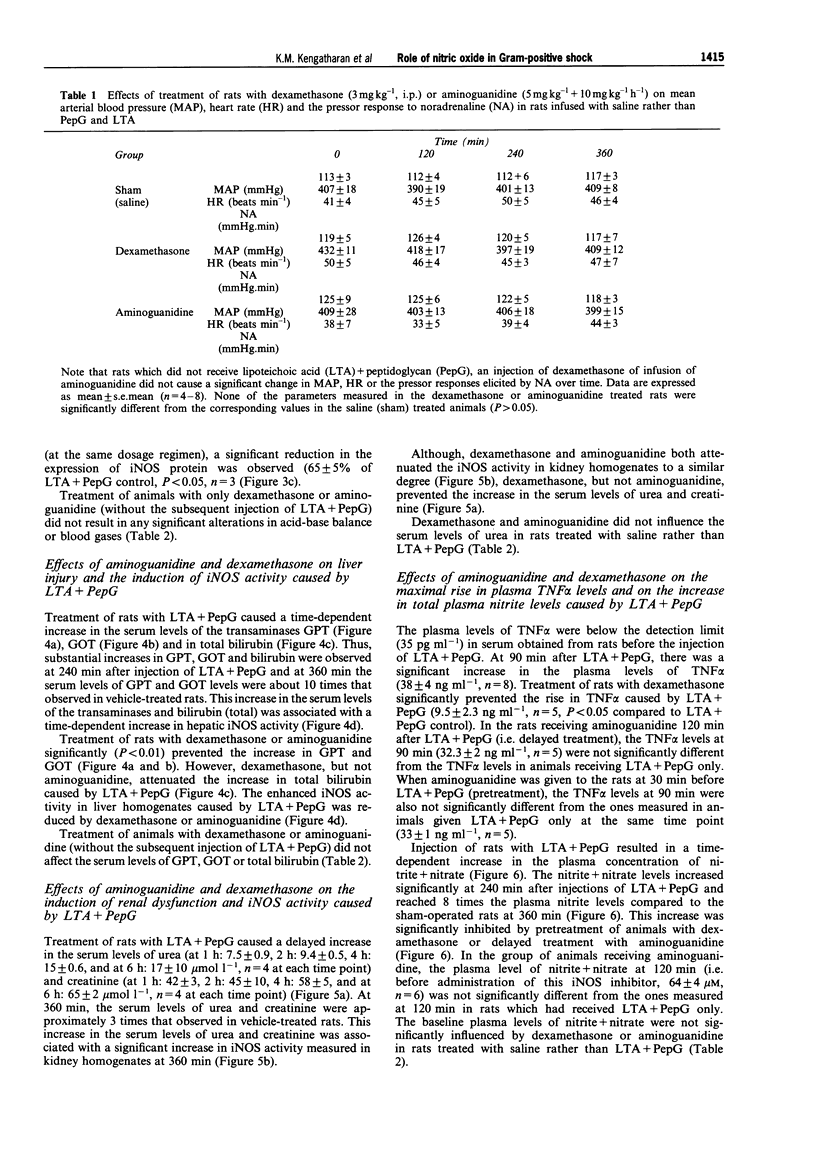
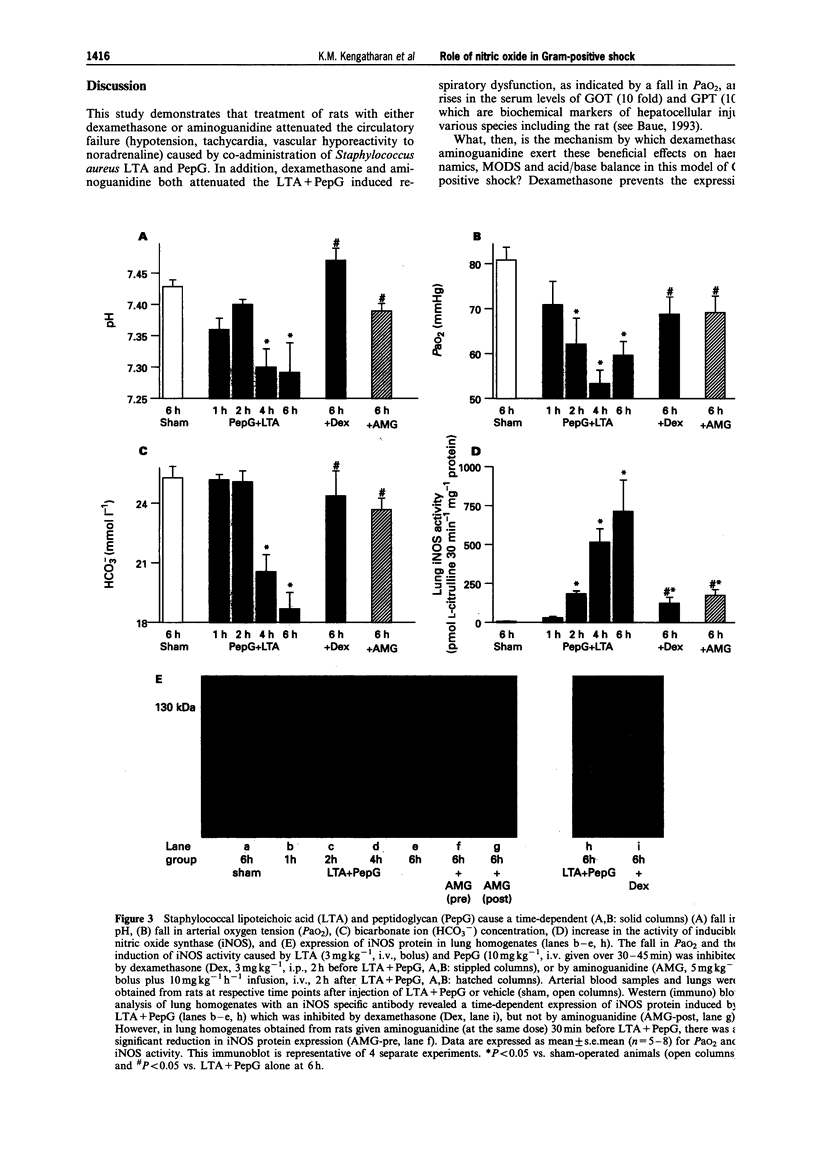
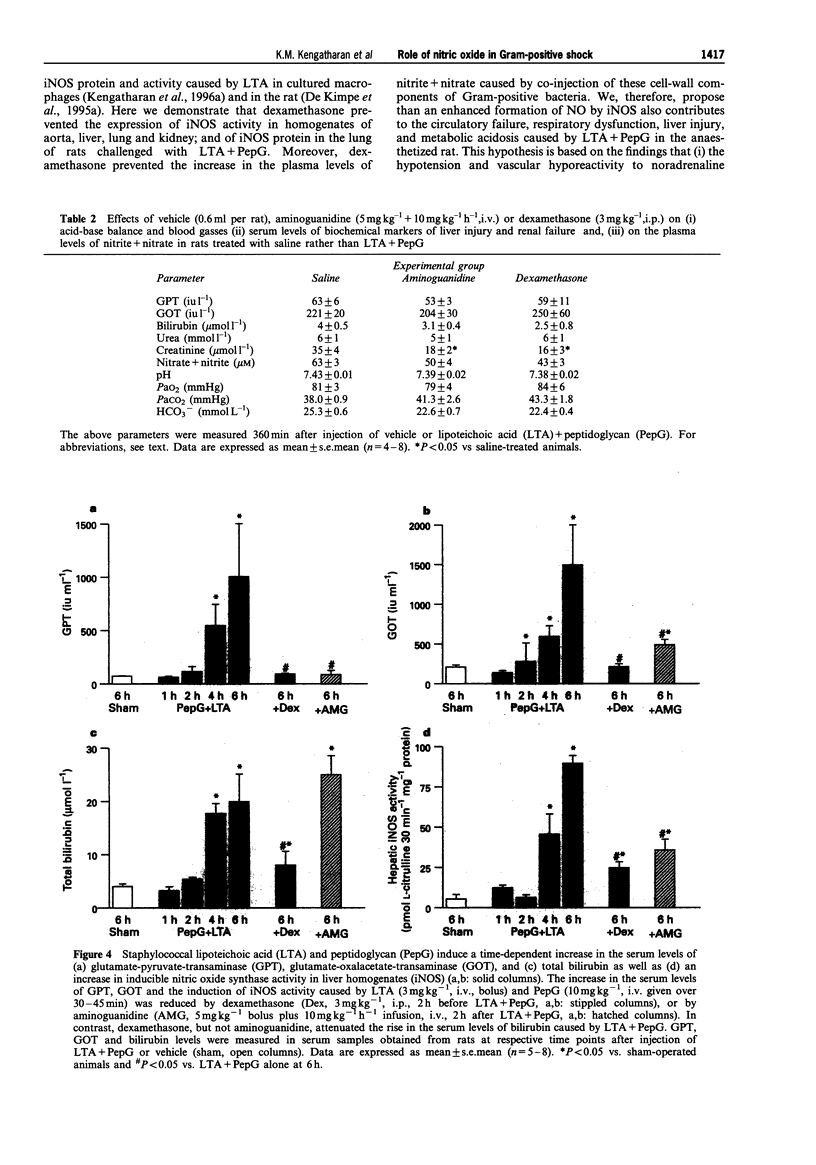
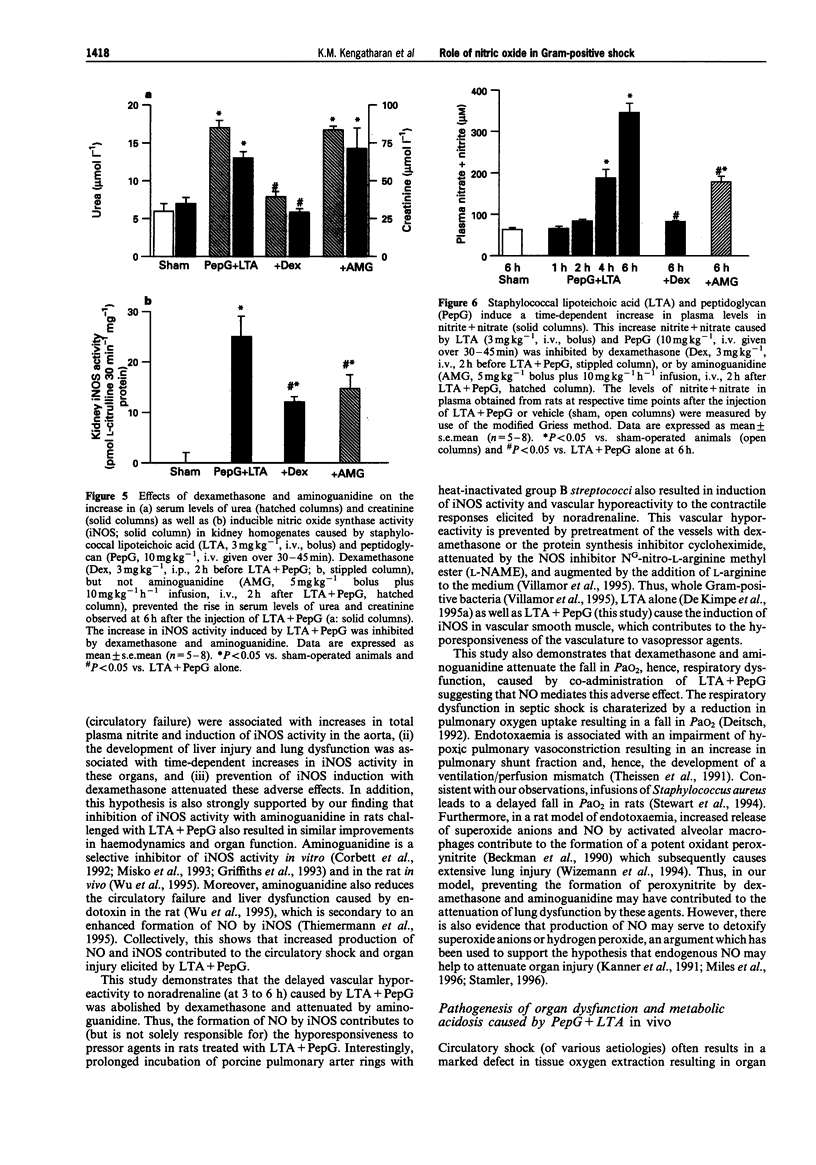
1. The pathological features of Gram-positive shock can be mimicked by the co-administration of two cell wall components of Staphylococcus aureus, namely lipoteichoic acid (LTA) and peptidoglycan (PepG). This is associated with the expression of the inducible isoform of nitric oxide synthase (iNOS) in various organs. We have investigated the effects of dexamethasone (which prevents the expression of iNOS protein) or aminoguanidine (an inhibitor of iNOS activity) on haemodynamics, multiple organ dysfunction syndrome (MODS) as well as iNOS activity elicited by LTA + PepG in anaesthetized rats. 2. Co-administration of LTA (3 mg kg-1, i.v.) and PepG (10 mg kg-1, i.v.) resulted in a significant increase in the plasma levels of tumour necrosis factor-alpha (TNF alpha, maximum at 90 min) as well as a biphasic fall in mean arterial blood pressure (MAP) from 120 +/- 3 mmHg (time 0) to 77 +/- 5 mmHg (at 6 h, n = 8; P < 0.05). This hypotension was associated with a significant tachycardia (4-6 h, P < 0.05) and a reduction of the pressor response elicited by noradrenaline (NA, 1 microgram kg-1, i.v., at 1-6 h; n = 8, P < 0.05). Furthermore, LTA + PepG caused time-dependent increases in the serum levels of markers of hepatocellular injury, glutamate-pyruvate-transminase (GPT) and glutamate-oxalacetate-transaminase (GOT). In addition, urea and creatinine (indicators of renal dysfunction) were increased. There was also a fall in arterial oxygen tension (PaO2), indicating respiratory dysfunction, and metabolic acidosis as shown by the significant drop in pH, PaCO2 and HCO3-. These effects caused by LTA + PepG were associated with the induction of iNOS activity in aorta, liver, kidney and lungs as well as increases in serum levels of nitrite+nitrate (total nitrite). 3. Pretreatment of rats with dexamethasone (3 mg kg-1, i.p.) at 120 min before LTA + PepG administration significantly attenuated these adverse effects as well as the increases in the plasma levels of TNF alpha caused by LTA + PepG. The protective effects of dexamethasone were associated with a prevention of the increase in iNOS activity (in aorta, liver, lung, kidney), the expression of iNOS protein (in lungs), as well as in the increase in the plasma levels of total nitrite. 4. Treatment of rats with aminoguanidine (5 mg kg-1 + 10 mg kg-1 h-1) starting at 120 min after LTA + PepG attenuated most of the adverse effects and gave a significant inhibition of iNOS activity (in various organs) as well as an inhibition of the increase in total plasma nitrite. However, aminoguanidine did not improve renal function although this agent caused a substantial inhibition of NOS activity in the kidney. 5. Thus, an enhanced formation of NO by iNOS importantly contributes to the circulatory failure, hepatocellular injury, respiratory dysfunction and the metabolic acidosis, but not the renal failure, caused by LTA + PepG in the anaesthetized rat.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auguet M., Lonchampt M. O., Delaflotte S., Goulin-Schulz J., Chabrier P. E., Braquet P. Induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in vascular smooth muscle cells. FEBS Lett. 1992 Feb 3;297(1-2):183–185. doi: 10.1016/0014-5793(92)80356-l. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Biegański T., Kusche J., Lorenz W., Hesterberg R., Stahlknecht C. D., Feussner K. D. Distribution and properties of human intestinal diamine oxidase and its relevance for the histamine catabolism. Biochim Biophys Acta. 1983 Mar 31;756(2):196–203. doi: 10.1016/0304-4165(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Bone R. C. Gram-positive organisms and sepsis. Arch Intern Med. 1994 Jan 10;154(1):26–34. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kimmel S. C., Levin R. I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha F. Q., Moss D. W., Leal L. M., Moncada S., Liew F. Y. Induction of macrophage parasiticidal activity by Staphylococcus aureus and exotoxins through the nitric oxide synthesis pathway. Immunology. 1993 Apr;78(4):563–567. [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Ferrari F. K., Kispert P. H., Stadler J., Stuehr D. J., Simmons R. L., Billiar T. R. Nitric oxide and nitric oxide-generating compounds inhibit hepatocyte protein synthesis. FASEB J. 1991 Apr;5(7):2085–2092. doi: 10.1096/fasebj.5.7.1707021. [DOI] [PubMed] [Google Scholar]
- De Kimpe S. J., Hunter M. L., Bryant C. E., Thiemermann C., Vane J. R. Delayed circulatory failure due to the induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in anaesthetized rats. Br J Pharmacol. 1995 Mar;114(6):1317–1323. doi: 10.1111/j.1476-5381.1995.tb13349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kimpe S. J., Kengatharan M., Thiemermann C., Vane J. R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch E. A. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992 Aug;216(2):117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein D., Brownlee M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes. 1992 Jan;41(1):26–29. doi: 10.2337/diab.41.1.26. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Eleventh Gaddum memorial lecture. Lipocortin and the mechanism of action of the glucocorticoids. Br J Pharmacol. 1988 Aug;94(4):987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. E., Pearlstein L., Fulton R. L., Polk H. C., Jr Multiple system organ failure. The role of uncontrolled infection. Arch Surg. 1980 Feb;115(2):136–140. doi: 10.1001/archsurg.1980.01380020006003. [DOI] [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M. J., Messent M., MacAllister R. J., Evans T. W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol. 1993 Nov;110(3):963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner J., Harel S., Granit R. Nitric oxide as an antioxidant. Arch Biochem Biophys. 1991 Aug 15;289(1):130–136. doi: 10.1016/0003-9861(91)90452-o. [DOI] [PubMed] [Google Scholar]
- Kanner J., Harel S., Granit R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids. 1992 Jan;27(1):46–49. doi: 10.1007/BF02537058. [DOI] [PubMed] [Google Scholar]
- Kengatharan M., De Kimpe S. J., Thiemermann C. Analysis of the signal transduction in the induction of nitric oxide synthase by lipoteichoic acid in macrophages. Br J Pharmacol. 1996 Mar;117(6):1163–1170. doi: 10.1111/j.1476-5381.1996.tb16711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepoivre M., Fieschi F., Coves J., Thelander L., Fontecave M. Inactivation of ribonucleotide reductase by nitric oxide. Biochem Biophys Res Commun. 1991 Aug 30;179(1):442–448. doi: 10.1016/0006-291x(91)91390-x. [DOI] [PubMed] [Google Scholar]
- Masferrer J. L., Seibert K., Zweifel B., Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. M., Bohle D. S., Glassbrenner P. A., Hansert B., Wink D. A., Grisham M. B. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem. 1996 Jan 5;271(1):40–47. doi: 10.1074/jbc.271.1.40. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Morris S. M., Jr, Billiar T. R. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994 Jun;266(6 Pt 1):E829–E839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Niemann A., Björklund A., Eizirik D. L. Studies on the molecular regulation of the inducible form of nitric oxide synthase (iNOS) in insulin-producing cells. Mol Cell Endocrinol. 1994 Dec;106(1-2):151–155. doi: 10.1016/0303-7207(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Ou P., Wolff S. P. Aminoguanidine: a drug proposed for prophylaxis in diabetes inhibits catalase and generates hydrogen peroxide in vitro. Biochem Pharmacol. 1993 Oct 5;46(7):1139–1144. doi: 10.1016/0006-2952(93)90461-5. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers N. E., Ignarro L. J. Constitutive nitric oxide synthase from cerebellum is reversibly inhibited by nitric oxide formed from L-arginine. Biochem Biophys Res Commun. 1992 Nov 30;189(1):242–249. doi: 10.1016/0006-291x(92)91550-a. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Nakane M., Förstermann U., Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992 Apr;41(4):615–624. [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B. The influence of catabolic reactions on polyamine excretion. Biochem J. 1985 Jan 1;225(1):219–226. doi: 10.1042/bj2250219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Harbrecht B. G., Di Silvio M., Curran R. D., Jordan M. L., Simmons R. L., Billiar T. R. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J Leukoc Biol. 1993 Feb;53(2):165–172. doi: 10.1002/jlb.53.2.165. [DOI] [PubMed] [Google Scholar]
- Stamler J. S. Alzheimer's disease. A radical vascular connection. Nature. 1996 Mar 14;380(6570):108–111. [PubMed] [Google Scholar]
- Stewart K. D., Brackett D. J., Lerner M. R., Archer L. T., Wilson M. F. Comparison of Staphylococcus aureus and Escherichia coli infusion in conscious rats. J Surg Res. 1994 Jan;56(1):60–66. doi: 10.1006/jsre.1994.1010. [DOI] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Thiemermann C., Wu C. C., Perretti M., Vane J. R. Attenuation of the induction of nitric oxide synthase by endogenous glucocorticoids accounts for endotoxin tolerance in vivo. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):271–275. doi: 10.1073/pnas.91.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen J. L., Loick H. M., Curry B. B., Traber L. D., Herndon D. N., Traber D. L. Time course of hypoxic pulmonary vasoconstriction after endotoxin infusion in unanesthetized sheep. J Appl Physiol (1985) 1991 May;70(5):2120–2125. doi: 10.1152/jappl.1991.70.5.2120. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Ruetten H., Wu C. C., Vane J. R. The multiple organ dysfunction syndrome caused by endotoxin in the rat: attenuation of liver dysfunction by inhibitors of nitric oxide synthase. Br J Pharmacol. 1995 Dec;116(7):2845–2851. doi: 10.1111/j.1476-5381.1995.tb15935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C. Selective inhibition of the activity of inducible nitric oxide synthase in septic shock. Prog Clin Biol Res. 1995;392:383–392. [PubMed] [Google Scholar]
- Thiemermann C., Wu C. C., Szabó C., Perretti M., Vane J. R. Role of tumour necrosis factor in the induction of nitric oxide synthase in a rat model of endotoxin shock. Br J Pharmacol. 1993 Sep;110(1):177–182. doi: 10.1111/j.1476-5381.1993.tb13789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamor E., Pérez-Vizcaíno F., Ruiz T., Leza J. C., Moro M., Tamargo J. Group B Streptococcus and E. coli LPS-induced NO-dependent hyporesponsiveness to noradrenaline in isolated intrapulmonary arteries of neonatal piglets. Br J Pharmacol. 1995 May;115(2):261–266. doi: 10.1111/j.1476-5381.1995.tb15872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz A., Cicatiello L., Esumi H. Regulation of the mouse inducible-type nitric oxide synthase gene promoter by interferon-gamma, bacterial lipopolysaccharide and NG-monomethyl-L-arginine. Biochem J. 1996 May 15;316(Pt 1):209–215. doi: 10.1042/bj3160209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D. A., Osawa Y., Darbyshire J. F., Jones C. R., Eshenaur S. C., Nims R. W. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys. 1993 Jan;300(1):115–123. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- Wizemann T. M., Gardner C. R., Laskin J. D., Quinones S., Durham S. K., Goller N. L., Ohnishi S. T., Laskin D. L. Production of nitric oxide and peroxynitrite in the lung during acute endotoxemia. J Leukoc Biol. 1994 Dec;56(6):759–768. doi: 10.1002/jlb.56.6.759. [DOI] [PubMed] [Google Scholar]
- Wu C. C., Chen S. J., Szabó C., Thiemermann C., Vane J. R. Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock. Br J Pharmacol. 1995 Apr;114(8):1666–1672. doi: 10.1111/j.1476-5381.1995.tb14955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Snyder S. H. Nitric oxide stimulates auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9382–9385. doi: 10.1073/pnas.89.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]