Abstract
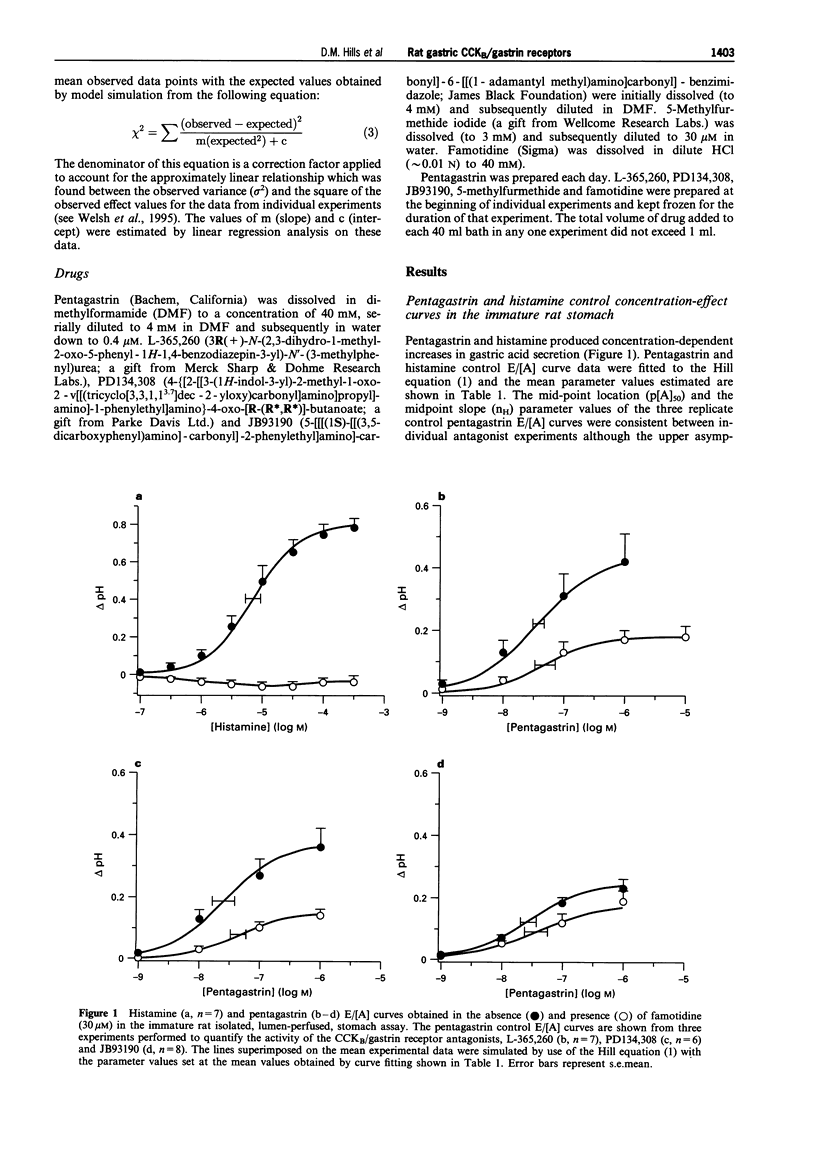
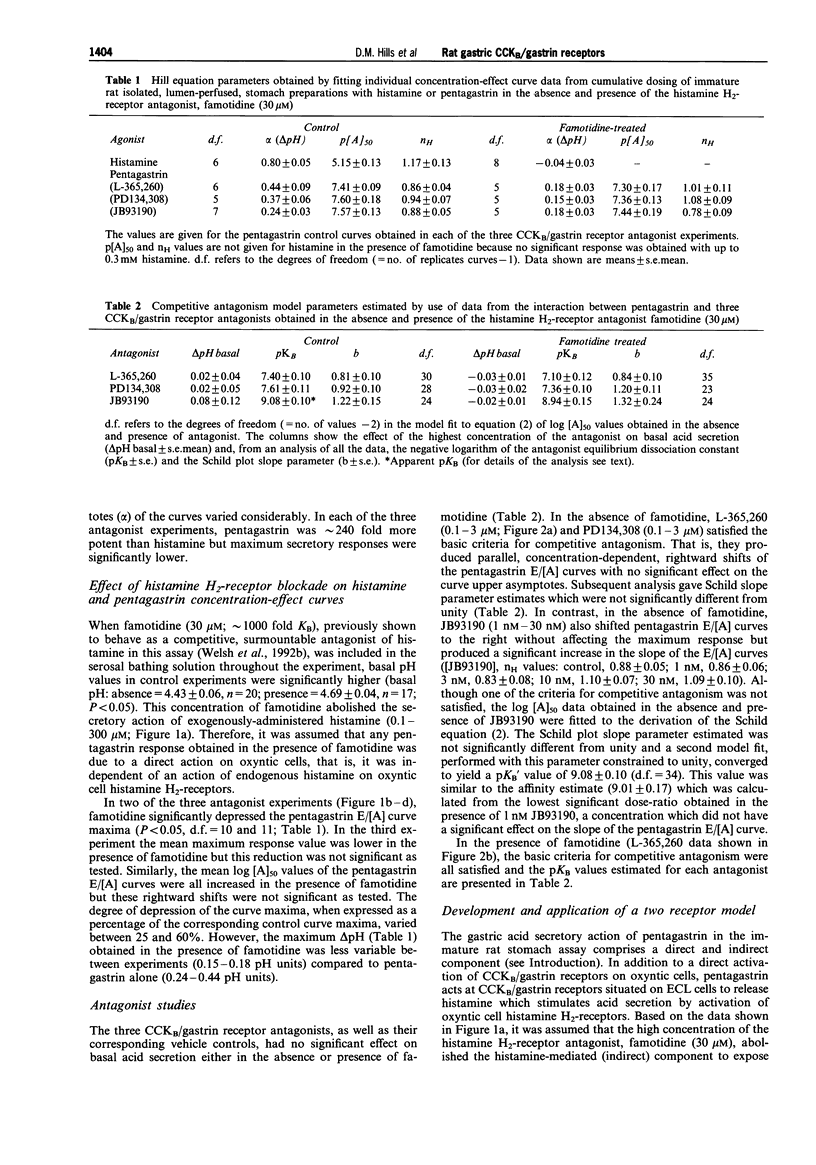
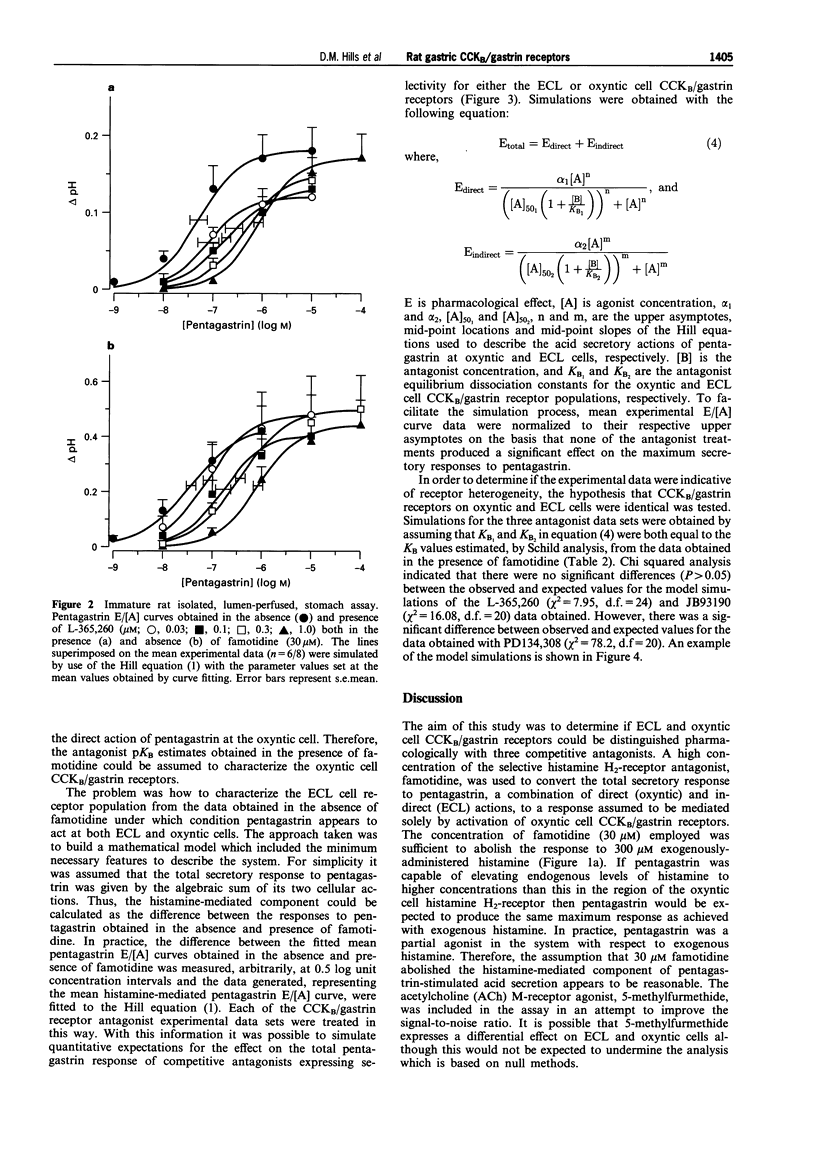
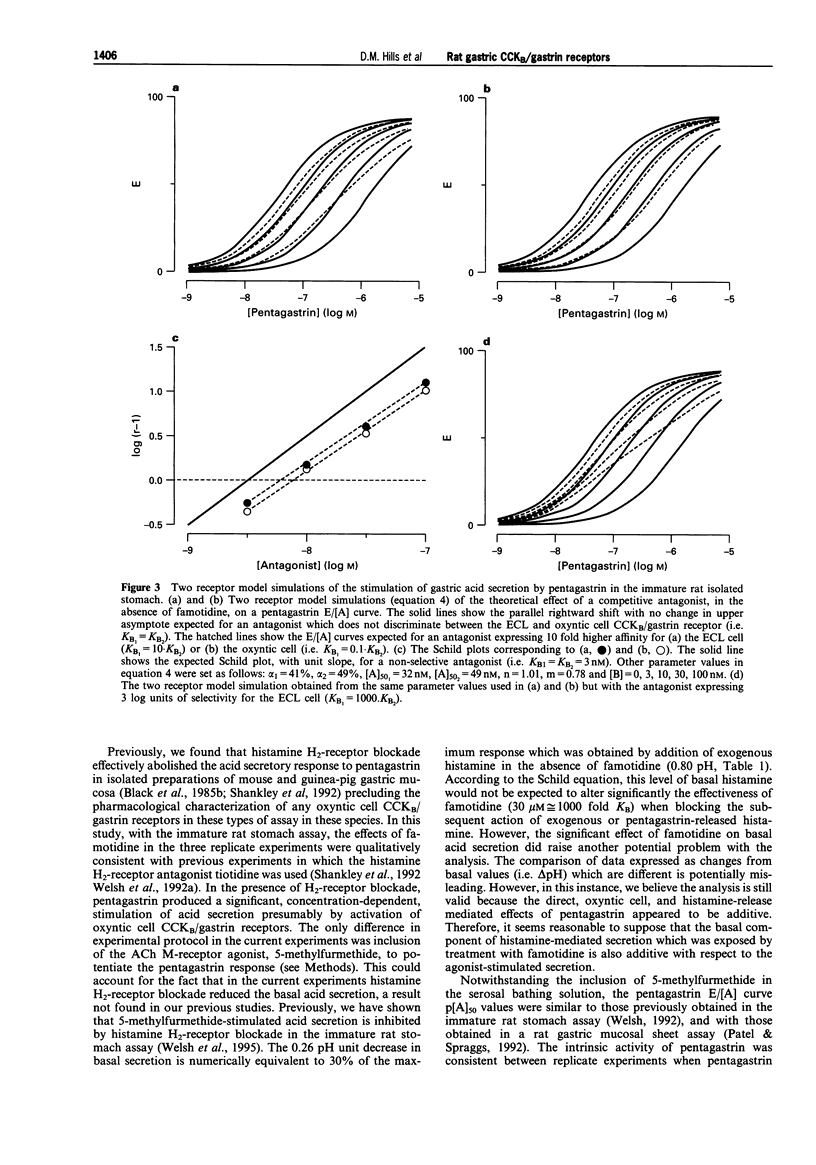
1. The CCKB/gastrin receptors mediating pentagastrin stimulation of gastric acid secretion by histamine release and by direct stimulation of oxyntic cells have been characterized in the immature rat isolated stomach assay. This was achieved by estimating antagonist affinity values for competitive antagonists from three distinct chemical classes (L-365,260, PD134,308 and JB93190) in the absence and presence of a high concentration of the histamine H2-receptor antagonist, famotidine (30 microM). 2. Pentagastrin produced concentration-dependent stimulation of gastric acid secretion in the absence and presence of famotidine. Famotidine depressed the maximum secretory response to pentagastrin although the degree of depression varied between experimental replicates (25-60%). This variation was attributed to the histamine-release mediated component of acid secretion, as judged by the consistency of the maximum responses obtained in the presence, but not absence, of famotidine. 3. All three CCKB/gastrin receptor antagonists behaved as surmountable antagonists in the absence and presence of famotidine. JB93190 (pKB approximately 9.1, approximately 8.9, in the absence and presence of famotidine, respectively) was approximately 30 fold more potent than either L-365,260 (pKB approximately 7.4, approximately 7.1) or PD134,308 (pKB approximately 7.6, approximately 7.4). 4. It was assumed that the famotidine treatment converted pentagastrin-stimulated acid secretion from a combination of an indirect action due to the release of histamine and a direct action on the oxyntic cell to solely a direct action on the oxyntic cell. A simple mathematical model of this two-receptor system was developed. The direct and indirect components were assumed to sum to produce the total response to pentagastrin obtained in the absence of famotidine. It was found that this model could account quantitatively for the behaviour of the three antagonists without invoking a difference in antagonist affinity for the CCKB/gastrin receptors mediating the direct and indirect actions of pentagastrin. However, a conclusion of receptor homogeneity has to be qualified because the model was also used to generate simulations which indicated that the analysis could only detect antagonist affinity differences of greater than one log-unit between enterochromaffin-like (ECL) and oxyntic cell CCKB/gastrin receptor populations.
Full text
PDF

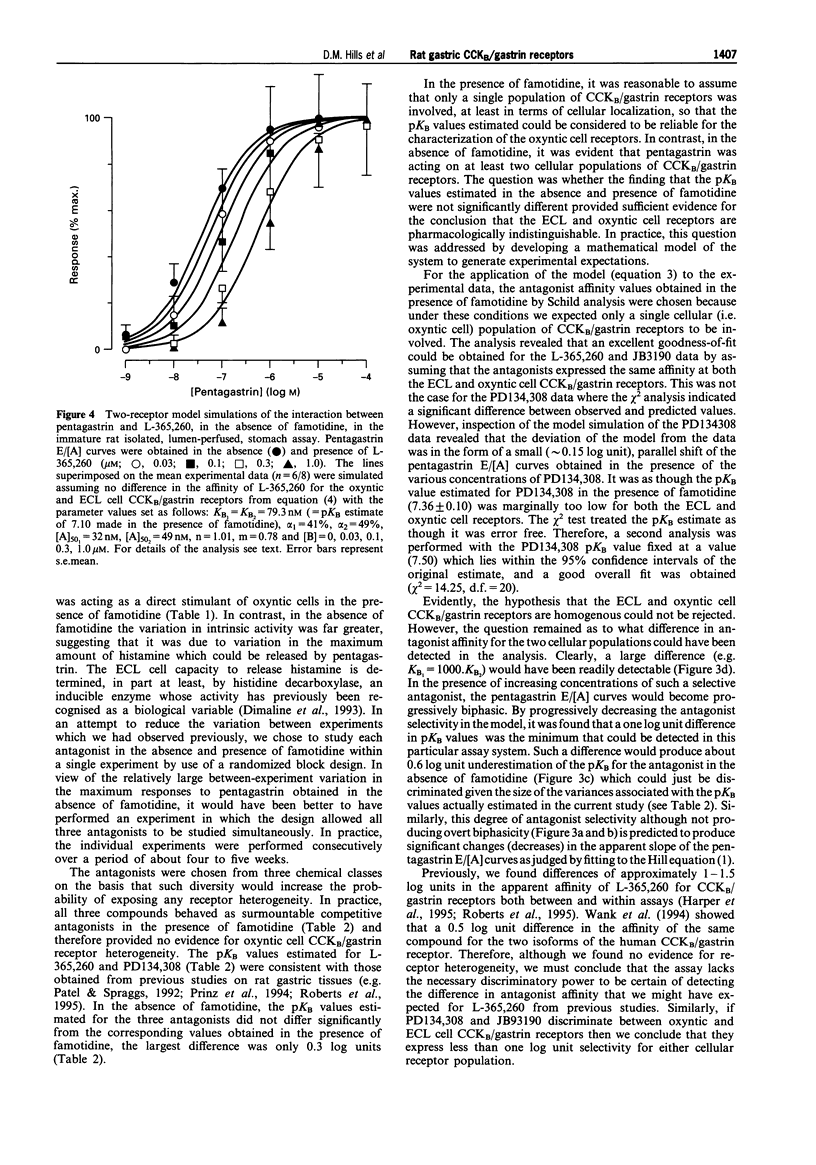
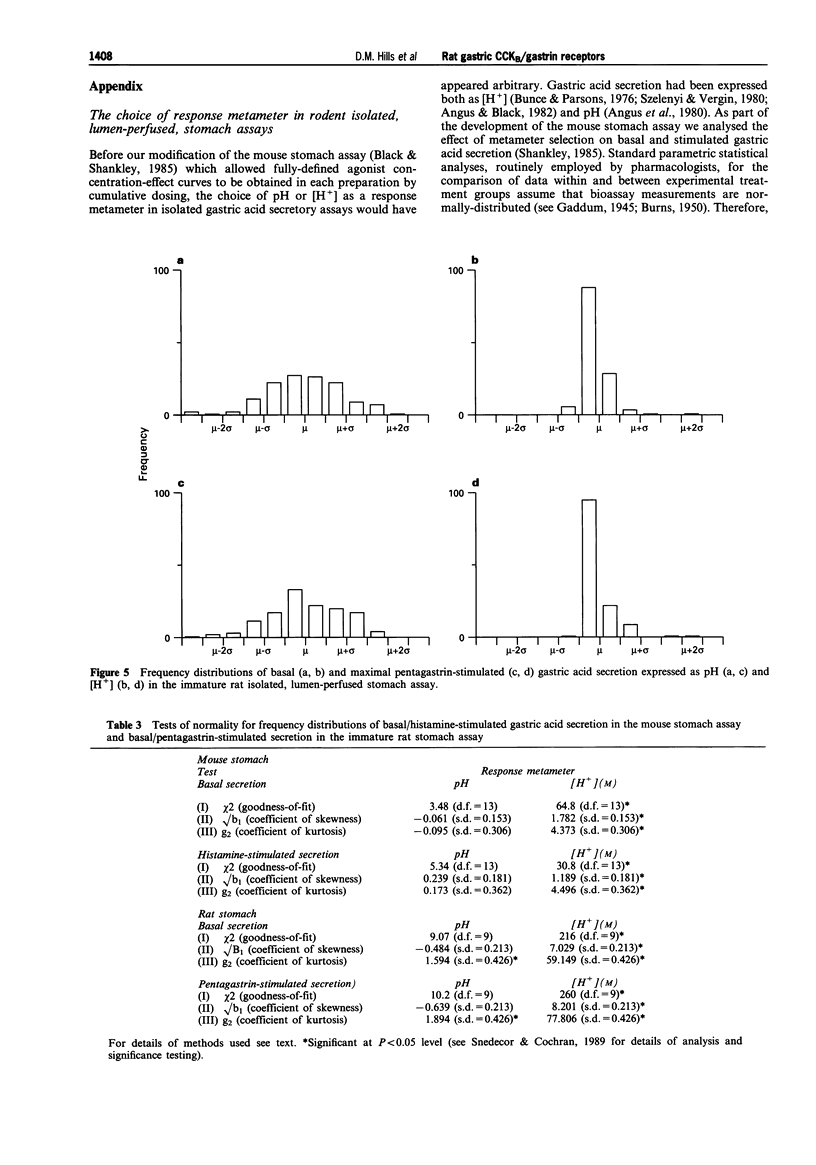


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Black J. W., Stone M. Estimation of pKB values for histamine H2-receptor antagonists using an in vitro acid secretion assay. Br J Pharmacol. 1980 Mar;68(3):413–423. doi: 10.1111/j.1476-5381.1980.tb14555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus J. A., Black J. W. The interaction of choline esters, vagal stimulation and H2-receptor blockade on acid secretion in vitro. Eur J Pharmacol. 1982 May 21;80(2-3):217–224. doi: 10.1016/0014-2999(82)90057-7. [DOI] [PubMed] [Google Scholar]
- Beinborn M., Lee Y. M., McBride E. W., Quinn S. M., Kopin A. S. A single amino acid of the cholecystokinin-B/gastrin receptor determines specificity for non-peptide antagonists. Nature. 1993 Mar 25;362(6418):348–350. doi: 10.1038/362348a0. [DOI] [PubMed] [Google Scholar]
- Black J. W., Leff P., Shankley N. P. Further analysis of anomalous pKB values for histamine H2-receptor antagonists on the mouse isolated stomach assay. Br J Pharmacol. 1985 Nov;86(3):581–587. doi: 10.1111/j.1476-5381.1985.tb08934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Leff P., Shankley N. P. Pharmacological analysis of the pentagastrin-tiotidine interaction in the mouse isolated stomach. Br J Pharmacol. 1985 Nov;86(3):589–599. doi: 10.1111/j.1476-5381.1985.tb08935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Shankley N. P. The isolated stomach preparation of the mouse: a physiological unit for pharmacological analysis. Br J Pharmacol. 1985 Nov;86(3):571–579. doi: 10.1111/j.1476-5381.1985.tb08933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandizzi C., Song I., Yamada T. Molecular cloning and structural analysis of the rabbit gastrin/CCKB receptor gene. Biochem Biophys Res Commun. 1994 Jul 29;202(2):947–953. doi: 10.1006/bbrc.1994.2021. [DOI] [PubMed] [Google Scholar]
- Bunce K. T., Parsons M. E. A quantitative study of metiamide, a histamine H2-antagonist, on the isolated whole rat stomach. J Physiol. 1976 Jun;258(2):453–465. doi: 10.1113/jphysiol.1976.sp011430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Boden P., Costall B., Domeney A., Kelly E., Horwell D. C., Hunter J. C., Pinnock R. D., Woodruff G. N. Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6728–6732. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Iwata N., Taniguchi T., Murayama T., Chihara K., Matsui T. Functional characterization of two cholecystokinin-B/gastrin receptor isoforms: a preferential splice donor site in the human receptor gene. Cell Growth Differ. 1994 Oct;5(10):1127–1135. [PubMed] [Google Scholar]
- Ito M., Matsui T., Taniguchi T., Tsukamoto T., Murayama T., Arima N., Nakata H., Chiba T., Chihara K. Functional characterization of a human brain cholecystokinin-B receptor. A trophic effect of cholecystokinin and gastrin. J Biol Chem. 1993 Aug 25;268(24):18300–18305. [PubMed] [Google Scholar]
- Jenkinson D. H. How we describe competitive antagonists: three questions of usage. Trends Pharmacol Sci. 1991 Feb;12(2):53–54. doi: 10.1016/0165-6147(91)90497-g. [DOI] [PubMed] [Google Scholar]
- Kalindjian S. B., Buck I. M., Davies J. M., Dunstone D. J., Hudson M. L., Low C. M., McDonald I. M., Pether M. J., Steel K. I., Tozer M. J. Non-peptide cholecystokinin-B/gastrin receptor antagonists based on bicyclic, heteroaromatic skeletons. J Med Chem. 1996 Apr 26;39(9):1806–1815. doi: 10.1021/jm9508907. [DOI] [PubMed] [Google Scholar]
- Kopin A. S., Lee Y. M., McBride E. W., Miller L. J., Lu M., Lin H. Y., Kolakowski L. F., Jr, Beinborn M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3605–3609. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. M., Beinborn M., McBride E. W., Lu M., Kolakowski L. F., Jr, Kopin A. S. The human brain cholecystokinin-B/gastrin receptor. Cloning and characterization. J Biol Chem. 1993 Apr 15;268(11):8164–8169. [PubMed] [Google Scholar]
- Lotti V. J., Chang R. S. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260. Eur J Pharmacol. 1989 Mar 21;162(2):273–280. doi: 10.1016/0014-2999(89)90290-2. [DOI] [PubMed] [Google Scholar]
- Nakata H., Matsui T., Ito M., Taniguchi T., Naribayashi Y., Arima N., Nakamura A., Kinoshita Y., Chihara K., Hosoda S. Cloning and characterization of gastrin receptor from ECL carcinoid tumor of Mastomys natalensis. Biochem Biophys Res Commun. 1992 Sep 16;187(2):1151–1157. doi: 10.1016/0006-291x(92)91317-j. [DOI] [PubMed] [Google Scholar]
- Patel M., Spraggs C. F. Functional comparisons of gastrin/cholecystokinin receptors in isolated preparations of gastric mucosa and ileum. Br J Pharmacol. 1992 Jun;106(2):275–282. doi: 10.1111/j.1476-5381.1992.tb14328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisegna J. R., de Weerth A., Huppi K., Wank S. A. Molecular cloning of the human brain and gastric cholecystokinin receptor: structure, functional expression and chromosomal localization. Biochem Biophys Res Commun. 1992 Nov 30;189(1):296–303. doi: 10.1016/0006-291x(92)91557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti M. E., Gardner J. D. Receptor antagonists for gastrointestinal peptides. Am J Physiol. 1993 Mar;264(3 Pt 1):G399–G406. doi: 10.1152/ajpgi.1993.264.3.G399. [DOI] [PubMed] [Google Scholar]
- Prinz C., Scott D. R., Hurwitz D., Helander H. F., Sachs G. Gastrin effects on isolated rat enterochromaffin-like cells in primary culture. Am J Physiol. 1994 Oct;267(4 Pt 1):G663–G675. doi: 10.1152/ajpgi.1994.267.4.G663. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Four basic characteristics of the gastrin-cholecystokinin system. Am J Physiol. 1981 Apr;240(4):G255–G266. doi: 10.1152/ajpgi.1981.240.4.G255. [DOI] [PubMed] [Google Scholar]
- Sandvik A. K., Waldum H. L., Kleveland P. M., Schulze Søgnen B. Gastrin produces an immediate and dose-dependent histamine release preceding acid secretion in the totally isolated, vascularly perfused rat stomach. Scand J Gastroenterol. 1987 Sep;22(7):803–808. doi: 10.3109/00365528708991918. [DOI] [PubMed] [Google Scholar]
- Savarese T. M., Fraser C. M. In vitro mutagenesis and the search for structure-function relationships among G protein-coupled receptors. Biochem J. 1992 Apr 1;283(Pt 1):1–19. doi: 10.1042/bj2830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankley N. P., Welsh N. J., Black J. W. Histamine dependence of pentagastrin-stimulated gastric acid secretion in rats. Yale J Biol Med. 1992 Nov-Dec;65(6):613–619. [PMC free article] [PubMed] [Google Scholar]
- Song I., Brown D. R., Wiltshire R. N., Gantz I., Trent J. M., Yamada T. The human gastrin/cholecystokinin type B receptor gene: alternative splice donor site in exon 4 generates two variant mRNAs. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9085–9089. doi: 10.1073/pnas.90.19.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelenyi I., Vergin H. Cholinergic pathway of gastric acid secretion in the isolated whole stomach of the mouse. Pharmacology. 1980;21(4):268–276. doi: 10.1159/000137441. [DOI] [PubMed] [Google Scholar]
- Wank S. A., Pisegna J. R., de Weerth A. Brain and gastrointestinal cholecystokinin receptor family: structure and functional expression. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8691–8695. doi: 10.1073/pnas.89.18.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh N. J., Shankley N. P., Black J. W. Application of a model to explore interspecies differences in acetylcholine M-receptor-stimulated gastric acid secretion. Br J Pharmacol. 1995 Jul;115(6):961–968. doi: 10.1111/j.1476-5381.1995.tb15904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh N. J., Shankley N. P., Black J. W. Comparative study of the control of basal acid output from rodent isolated stomachs. Br J Pharmacol. 1993 Aug;109(4):941–945. doi: 10.1111/j.1476-5381.1993.tb13711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff G. N., Hughes J. Cholecystokinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:469–501. doi: 10.1146/annurev.pa.31.040191.002345. [DOI] [PubMed] [Google Scholar]


