Abstract
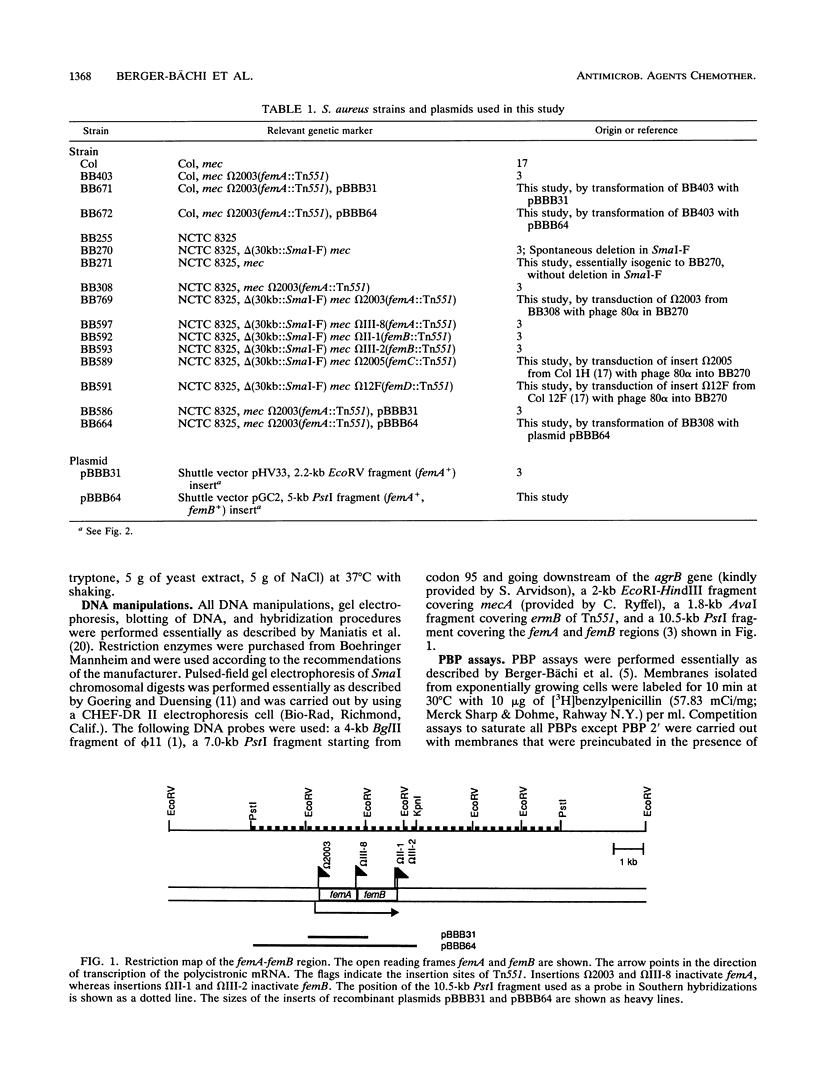
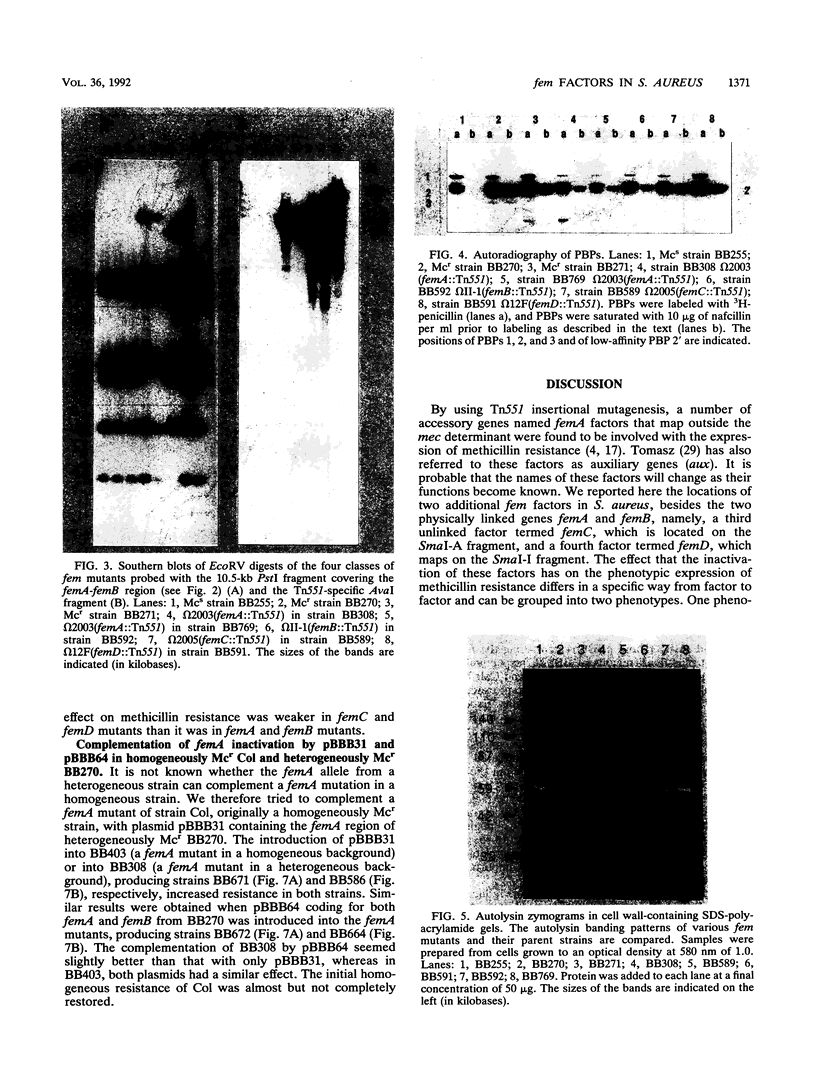
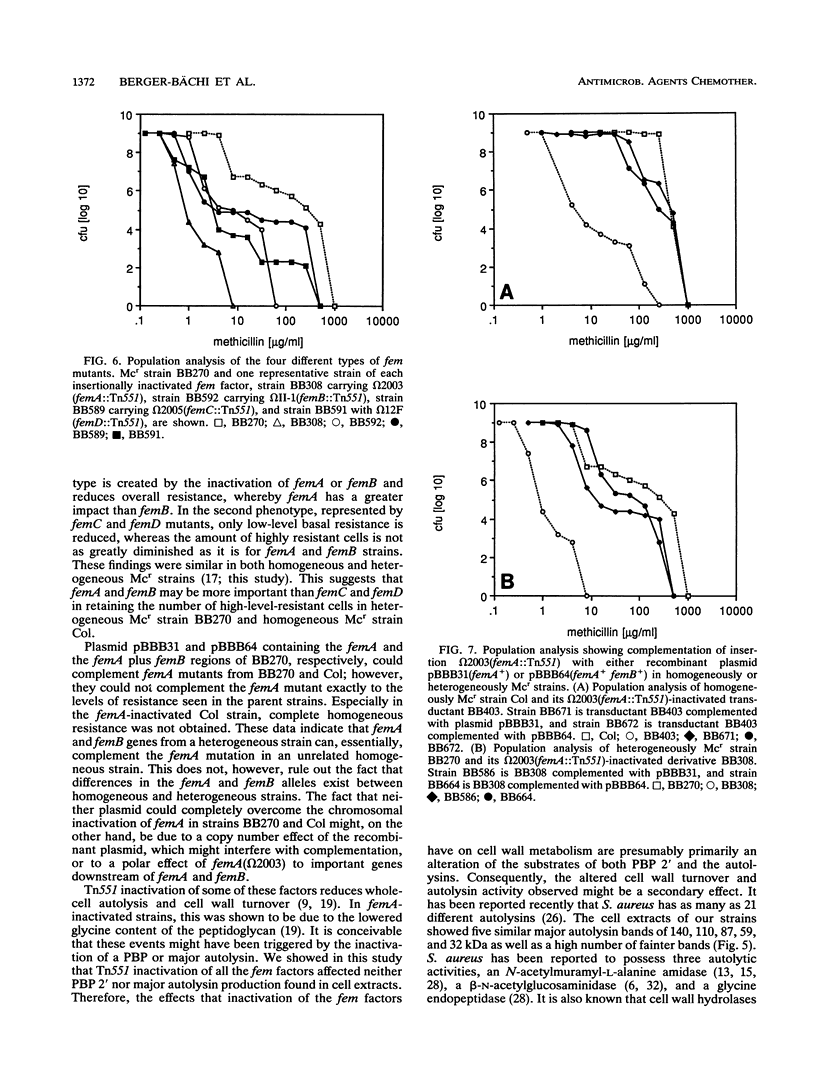
Chromosomal factors, termed fem or aux factors, are needed for the expression of methicillin resistance in methicillin-resistant (Mcr) Staphylococcus aureus; also needed is the mec-encoded low-affinity penicillin-binding protein PBP 2'. These factors make up part of the normal set of genes present in susceptible and resistant strains of S. aureus and can be identified by Tn551-mediated insertional inactivation of the methicillin resistance. In this study, we characterized different Tn551 inserts and mapped them into four distinct loci on the SmaI chromosomal map of S. aureus NCTC 8325, thereby identifying two new loci which code for fem factors. The largest fragment, SmaI-A, carries three loci, two coding for both closely linked factors femA and femB and a novel third locus (femC) that is not linked to the other two. An additional, fourth, locus, femD, was identified in fragment SmaI-I. femA and femB inactivation reduced overall methicillin resistance, whereby femB had less of an influence on the resistance level. femC and femD inactivation reduced mainly the basal resistance level in heterogeneously Mcr strains and had less of an impact on the subpopulation with high-level resistance. Inactivation of either of these factors was shown to have no influence on the production of PBP 2', the main factor mediating methicillin resistance. In addition, no changes were observed in the banding patterns of the major autolysins in whole-cell extracts of the fem mutants, suggesting that the reduced cell wall turnover and autolysis observed in some of the insertionally inactivated strains were due to changes either of the substrate or in the autolysin control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger-Bächi B., Barberis-Maino L., Strässle A., Kayser F. H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989 Oct;219(1-2):263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983 Apr;154(1):479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B., Strässle A., Kayser F. H. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986 Dec;5(6):697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- Bächi B. Physical mapping of the BglI, BglII, PstI and EcoRI restriction fragments of staphylococcal phage phi 11 DNA. Mol Gen Genet. 1980;180(2):391–398. doi: 10.1007/BF00425853. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Hackbarth C. J. Effect of NaCl and nafcillin on penicillin-binding protein 2a and heterogeneous expression of methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Dec;31(12):1982–1988. doi: 10.1128/aac.31.12.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F. Methicillin-resistant staphylococci. Clin Microbiol Rev. 1988 Apr;1(2):173–186. doi: 10.1128/cmr.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R. Penicillin-binding proteins and the intrinsic resistance to beta-lactams in gram-positive cocci. J Antimicrob Chemother. 1985 Oct;16(4):412–416. doi: 10.1093/jac/16.4.412. [DOI] [PubMed] [Google Scholar]
- Goering R. V., Duensing T. D. Rapid field inversion gel electrophoresis in combination with an rRNA gene probe in the epidemiological evaluation of staphylococci. J Clin Microbiol. 1990 Mar;28(3):426–429. doi: 10.1128/jcm.28.3.426-429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff E., Silverman C. S., Adams N. J., Awkard W. S. Extracellular cell wall lytic enzyme from Staphylococcus aureus: purification and partial characterization. J Bacteriol. 1970 Sep;103(3):761–769. doi: 10.1128/jb.103.3.761-769.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis B., Matthews P. R., Stewart P. R. The expression in Staphylococcus aureus of cloned DNA encoding methicillin resistance. J Gen Microbiol. 1988 Jun;134(6):1465–1469. doi: 10.1099/00221287-134-6-1465. [DOI] [PubMed] [Google Scholar]
- Jayaswal R. K., Lee Y. I., Wilkinson B. J. Cloning and expression of a Staphylococcus aureus gene encoding a peptidoglycan hydrolase activity. J Bacteriol. 1990 Oct;172(10):5783–5788. doi: 10.1128/jb.172.10.5783-5788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. Extracellular proteases modify cell wall turnover in Bacillus subtilis. J Bacteriol. 1980 Mar;141(3):1199–1208. doi: 10.1128/jb.141.3.1199-1208.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum J., Hartman B. J., Novick R. P., Tomasz A. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur J Clin Microbiol. 1986 Dec;5(6):714–718. doi: 10.1007/BF02013311. [DOI] [PubMed] [Google Scholar]
- Madiraju M. V., Brunner D. P., Wilkinson B. J. Effects of temperature, NaCl, and methicillin on penicillin-binding proteins, growth, peptidoglycan synthesis, and autolysis in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Nov;31(11):1727–1733. doi: 10.1128/aac.31.11.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidhof H., Reinicke B., Blümel P., Berger-Bächi B., Labischinski H. femA, which encodes a factor essential for expression of methicillin resistance, affects glycine content of peptidoglycan in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J Bacteriol. 1991 Jun;173(11):3507–3513. doi: 10.1128/jb.173.11.3507-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Song M. D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986 Sep;167(3):975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. H., Foster T. J., Pattee P. A. Physical and genetic mapping of the protein A gene in the chromosome of Staphylococcus aureus 8325-4. J Gen Microbiol. 1989 Jul;135(7):1799–1807. doi: 10.1099/00221287-135-7-1799. [DOI] [PubMed] [Google Scholar]
- Potvin C., Leclerc D., Tremblay G., Asselin A., Bellemare G. Cloning, sequencing and expression of a Bacillus bacteriolytic enzyme in Escherichia coli. Mol Gen Genet. 1988 Oct;214(2):241–248. doi: 10.1007/BF00337717. [DOI] [PubMed] [Google Scholar]
- Sugai M., Akiyama T., Komatsuzawa H., Miyake Y., Suginaka H. Characterization of sodium dodecyl sulfate-stable Staphylococcus aureus bacteriolytic enzymes by polyacrylamide gel electrophoresis. J Bacteriol. 1990 Nov;172(11):6494–6498. doi: 10.1128/jb.172.11.6494-6498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch W., Strässle A., Berger-Bächi B., O'Hara D., Reynolds P., Kayser F. H. Cloning and expression of methicillin resistance from Staphylococcus epidermidis in Staphylococcus carnosus. Antimicrob Agents Chemother. 1988 Oct;32(10):1494–1499. doi: 10.1128/aac.32.10.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Nachman S., Leaf H. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1991 Jan;35(1):124–129. doi: 10.1128/aac.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Nonoguchi R., Matsuhashi M., Konno M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989 May;171(5):2882–2885. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T., Hisatsune K. Bacteriolytic enzymes from Staphylococcus aureus. Purification of an endo-beta-N-acetylglucosaminidase. Biochem J. 1970 Dec;120(4):725–734. doi: 10.1042/bj1200725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge B. L., de Lencastre H., Tomasz A. Suppression of autolysis and cell wall turnover in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J Bacteriol. 1991 Feb;173(3):1105–1110. doi: 10.1128/jb.173.3.1105-1110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]






