Abstract
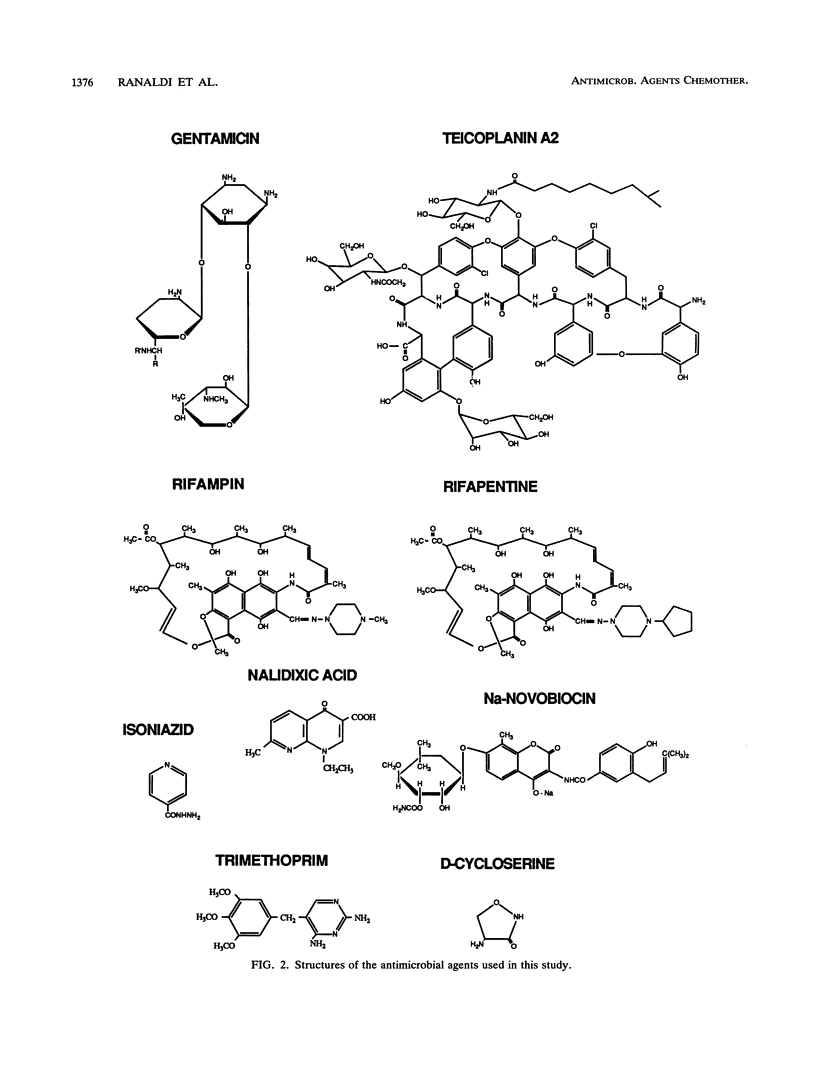
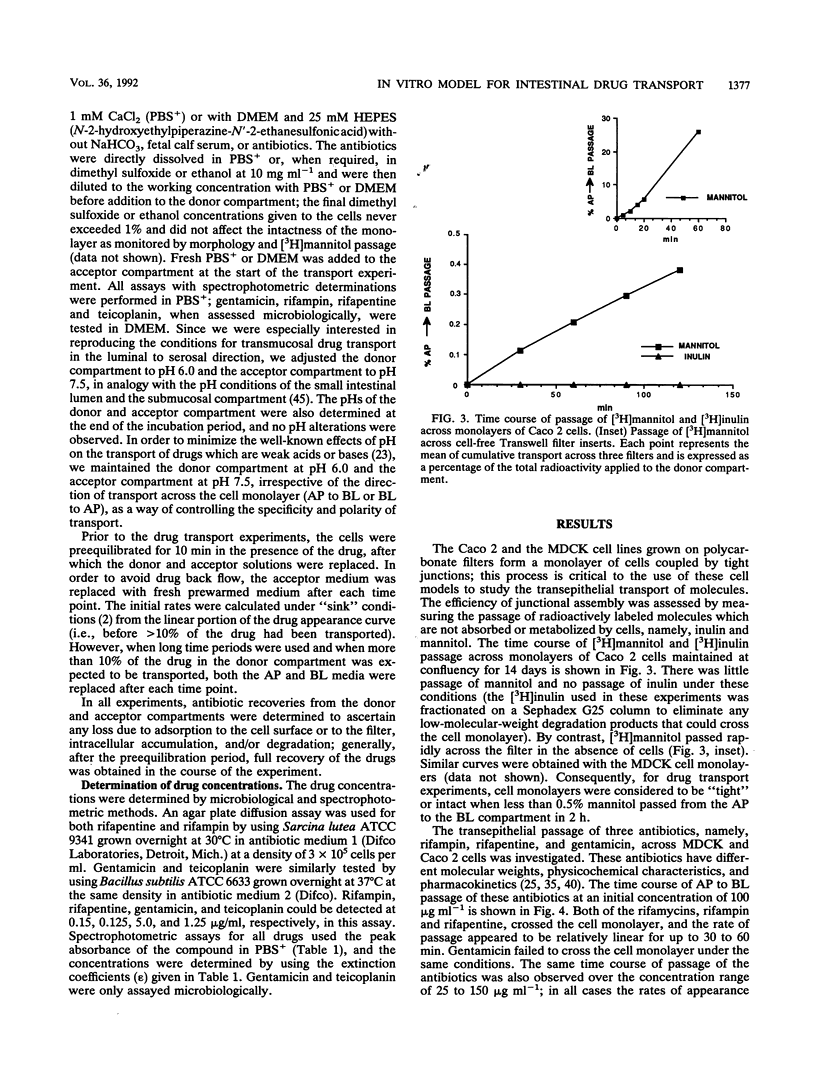
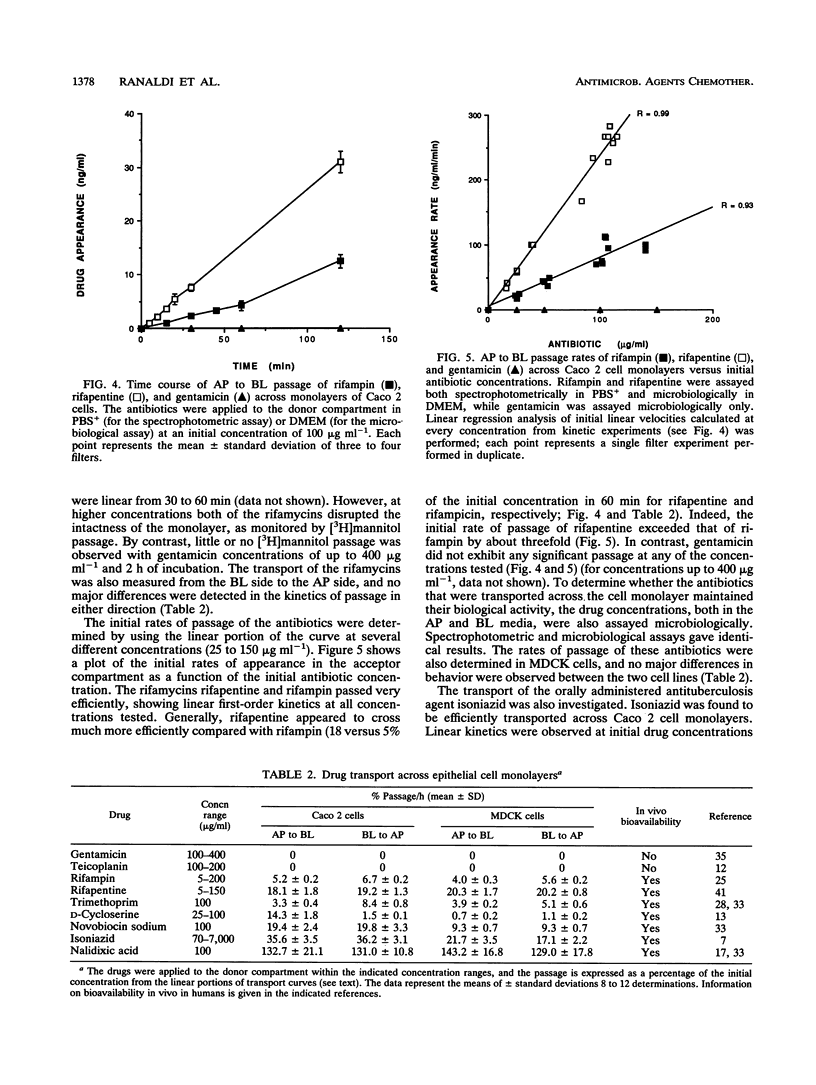
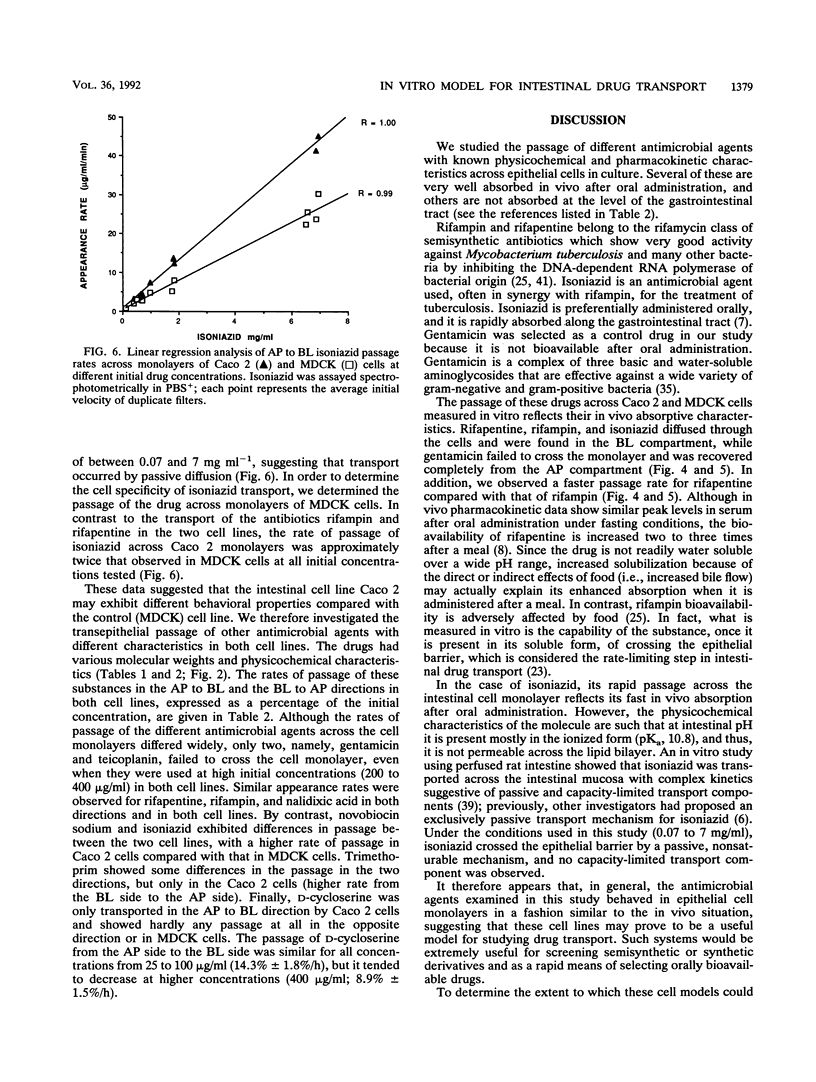
The bioavailabilities of orally administered drugs depend to a great extent on their capability of being transported across the intestinal mucosa. In an attempt to develop an in vitro model for studying the intestinal transport of drugs, we used an intestinal epithelial cell line (Caco 2) derived from a human colon adenocarcinoma. A renal epithelial cell line (MDCK) was also used to determine the tissue specificity of drug transport. These cell lines, which were grown on filters, form a monolayer of well-polarized cells coupled by tight junctions and can be used for transcellular transport experiments. We studied the transport of nine antimicrobial agents with different physicochemical and pharmacokinetic characteristics using these epithelial cell monolayers to determine whether this model could be predictive of oral bioavailability. The transepithelial passage was assayed from the apical (AP) to the basolateral (BL) side and in the opposite direction (BL to AP) in both cell lines. Radioactively labeled mannitol was used to monitor the intactness of the cell monolayer during drug passage. The results indicated that all antimicrobial agents tested tended to behave in vitro generally according to their known in vivo absorptive characteristics. In addition, the use of epithelia from different tissues enabled us to divide the drugs into four groups according to their behaviors and suggested the existence of different transport mechanisms. In particular, two antibiotics, gentamicin and teicoplanin, showed no passage in either direction or cell line, in accordance with their very poor in vivo absorbances after oral administration. In contrast, rifapentine, rifampin, and nalidixic acid passed very efficiently at similar rates in both directions and cell lines in a concentration-dependent, nonsaturable manner, which is suggestive of passive diffusion down a concentration gradient. Of the remaining drugs, isoniazid and novobiocin sodium showed some differences in passage between the two cell lines and, given their ionized state at the pH that was used, may use the paracellular route. Finally, trimethoprim and D-cycloserine exhibited differences in passage both with respect to polarity and cell line; in particular, trimethoprim had a faster rate of passage only in Caco 2 cells and in the BL to AP direction, while D-cycloserine was exclusively transported by Caco 2 cells in the AP to BL direction. In both cases it is possible that active transport mechanisms are involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Van Itallie C. M., Peterson M. D., Stevenson B. R., Carew E. A., Mooseker M. S. ZO-1 mRNA and protein expression during tight junction assembly in Caco-2 cells. J Cell Biol. 1989 Sep;109(3):1047–1056. doi: 10.1083/jcb.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artursson P. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J Pharm Sci. 1990 Jun;79(6):476–482. doi: 10.1002/jps.2600790604. [DOI] [PubMed] [Google Scholar]
- Artursson P., Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991 Mar 29;175(3):880–885. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- Artursson P., Magnusson C. Epithelial transport of drugs in cell culture. II: Effect of extracellular calcium concentration on the paracellular transport of drugs of different lipophilicities across monolayers of intestinal epithelial (Caco-2) cells. J Pharm Sci. 1990 Jul;79(7):595–600. doi: 10.1002/jps.2600790710. [DOI] [PubMed] [Google Scholar]
- Audus K. L., Bartel R. L., Hidalgo I. J., Borchardt R. T. The use of cultured epithelial and endothelial cells for drug transport and metabolism studies. Pharm Res. 1990 May;7(5):435–451. doi: 10.1023/a:1015800312910. [DOI] [PubMed] [Google Scholar]
- Barley J. F., Evered D. F., Troman S. M. Transport of isoniazid across rat small intestine in vitro. Biochem Pharmacol. 1972 Oct 1;21(19):2660–2661. doi: 10.1016/0006-2952(72)90237-7. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Dantzig A. H., Bergin L. Uptake of the cephalosporin, cephalexin, by a dipeptide transport carrier in the human intestinal cell line, Caco-2. Biochim Biophys Acta. 1990 Sep 7;1027(3):211–217. doi: 10.1016/0005-2736(90)90309-c. [DOI] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Grasset E., Pinto M., Dussaulx E., Zweibaum A., Desjeux J. F. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984 Sep;247(3 Pt 1):C260–C267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- Hidalgo I. J., Borchardt R. T. Transport of a large neutral amino acid (phenylalanine) in a human intestinal epithelial cell line: Caco-2. Biochim Biophys Acta. 1990 Sep 21;1028(1):25–30. doi: 10.1016/0005-2736(90)90261-l. [DOI] [PubMed] [Google Scholar]
- Hidalgo I. J., Borchardt R. T. Transport of bile acids in a human intestinal epithelial cell line, Caco-2. Biochim Biophys Acta. 1990 Jul 20;1035(1):97–103. doi: 10.1016/0304-4165(90)90179-z. [DOI] [PubMed] [Google Scholar]
- Hidalgo I. J., Raub T. J., Borchardt R. T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989 Mar;96(3):736–749. [PubMed] [Google Scholar]
- Hilgers A. R., Conradi R. A., Burton P. S. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm Res. 1990 Sep;7(9):902–910. doi: 10.1023/a:1015937605100. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Leighton J., Brada Z., Estes L. W., Justh G. Secretory activity and oncogenicity of a cell line (MDCK) derived from canine kidney. Science. 1969 Jan 31;163(3866):472–473. doi: 10.1126/science.163.3866.472. [DOI] [PubMed] [Google Scholar]
- Meza I., Ibarra G., Sabanero M., Martínez-Palomo A., Cereijido M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):746–754. doi: 10.1083/jcb.87.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Traber M. G., Goldberg I., Davidson E., Lagmay N., Kayden H. J. Vitamin E uptake by human intestinal cells during lipolysis in vitro. Gastroenterology. 1990 Jan;98(1):96–103. doi: 10.1016/0016-5085(90)91296-i. [DOI] [PubMed] [Google Scholar]
- Vincent M. L., Russell R. M., Sasak V. Folic acid uptake characteristics of a human colon carcinoma cell line, Caco-2. A newly-described cellular model for small intestinal epithelium. Hum Nutr Clin Nutr. 1985 Sep;39(5):355–360. [PubMed] [Google Scholar]
- Wilson G. Cell culture techniques for the study of drug transport. Eur J Drug Metab Pharmacokinet. 1990 Apr-Jun;15(2):159–163. doi: 10.1007/BF03190199. [DOI] [PubMed] [Google Scholar]


