Abstract
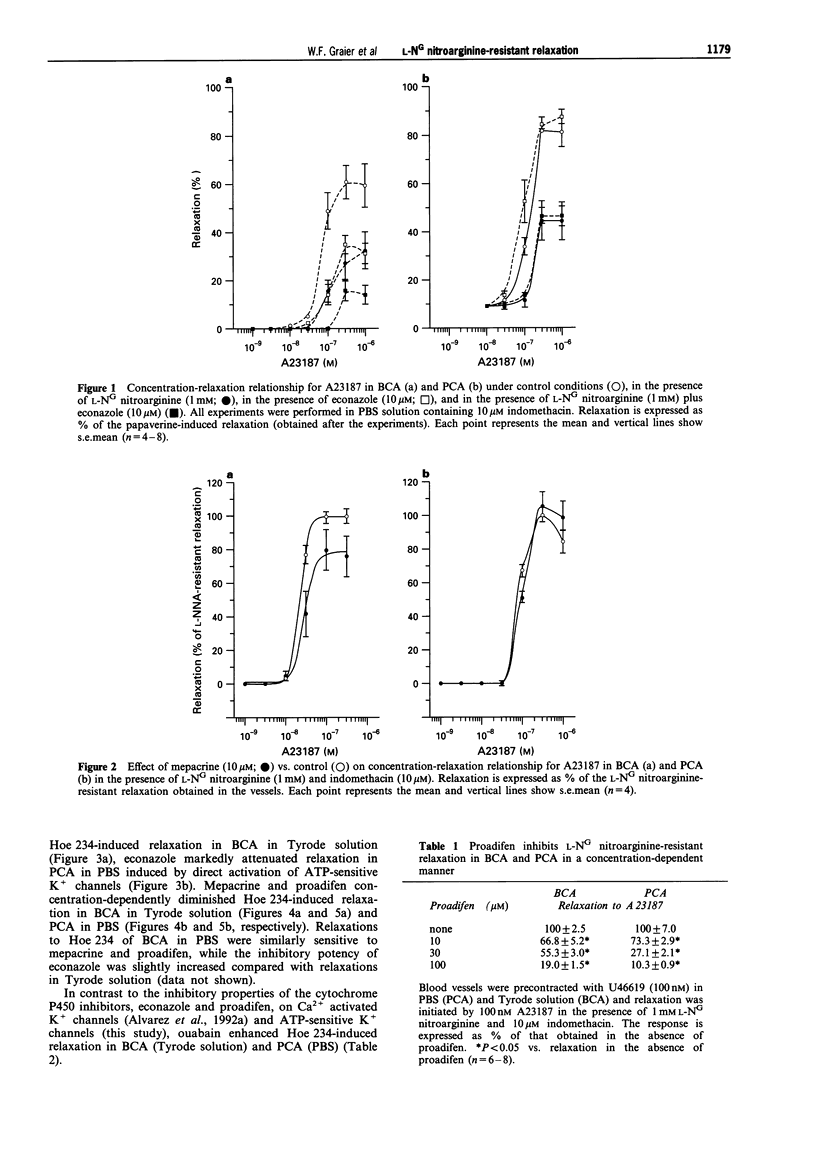
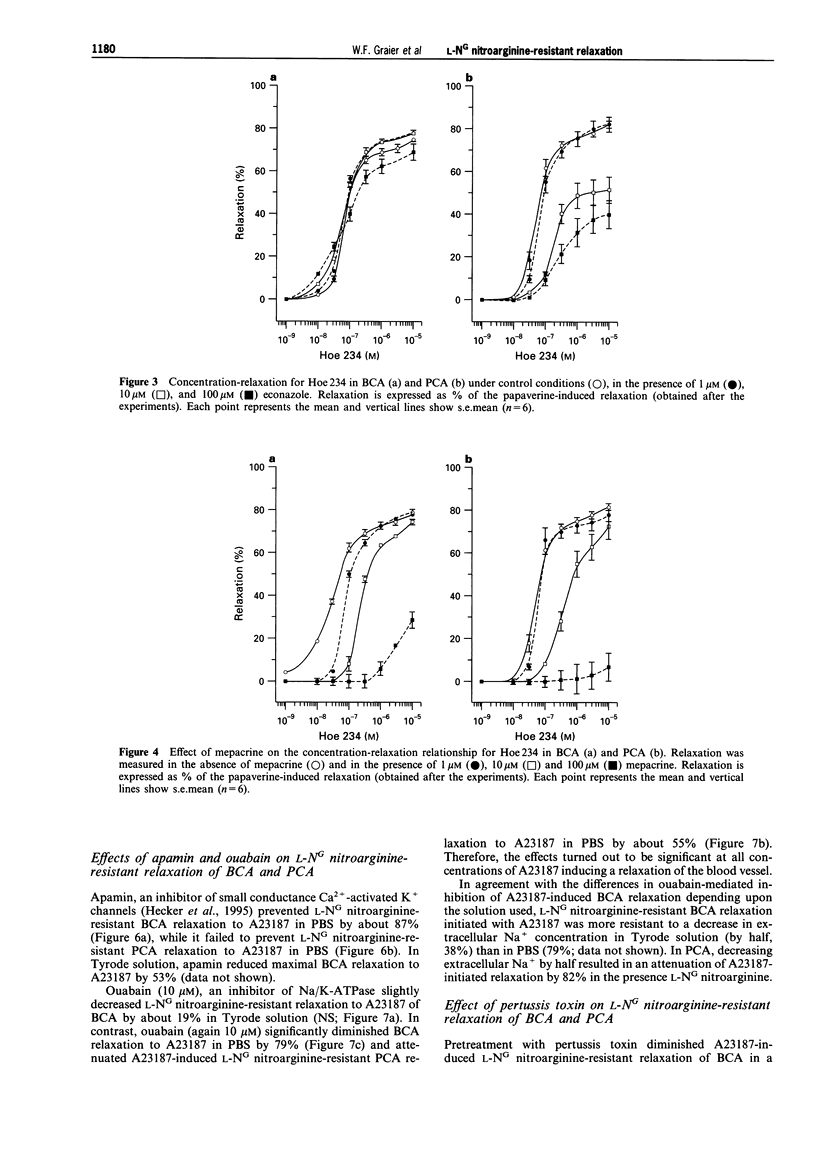
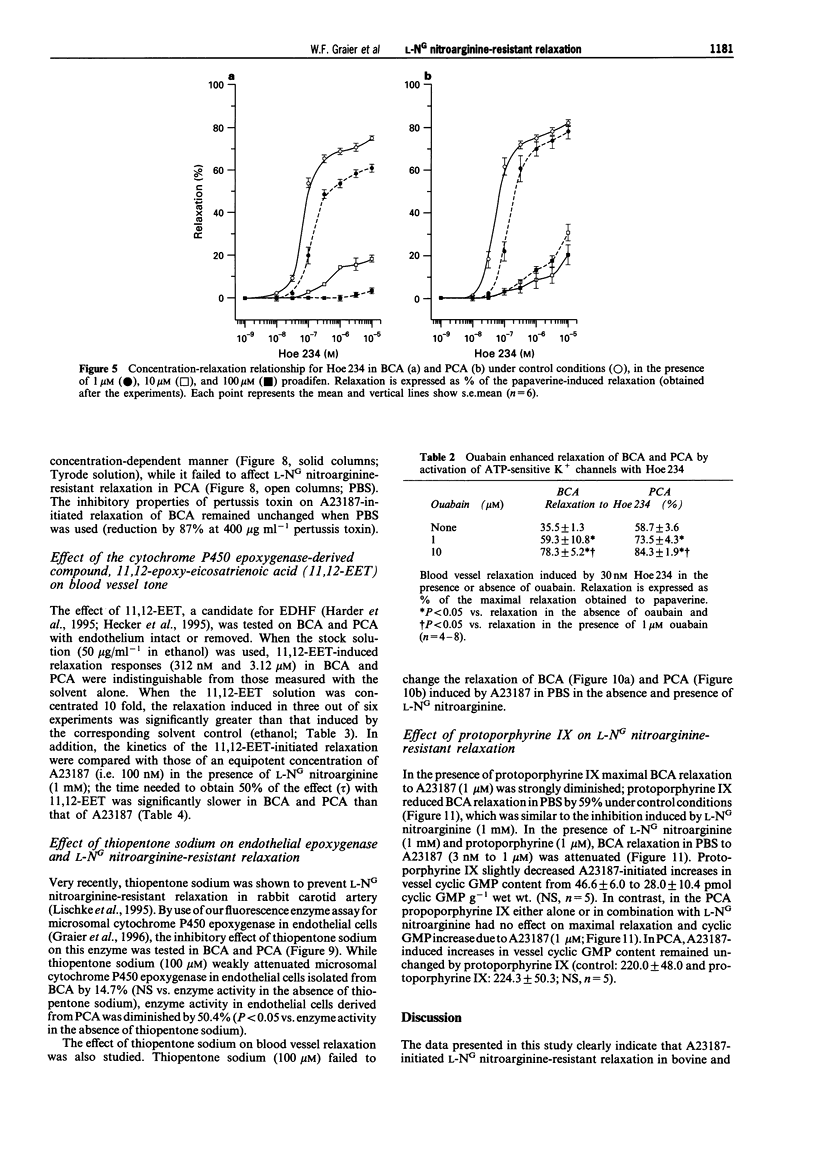
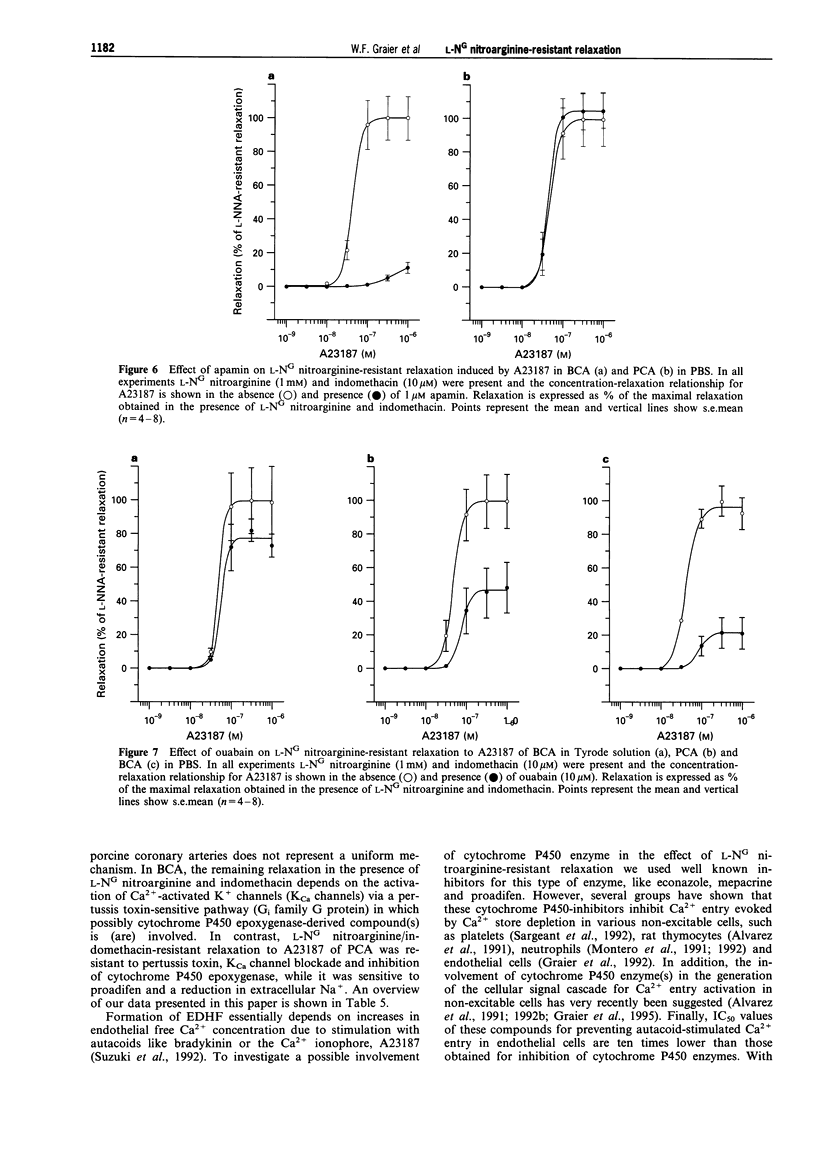
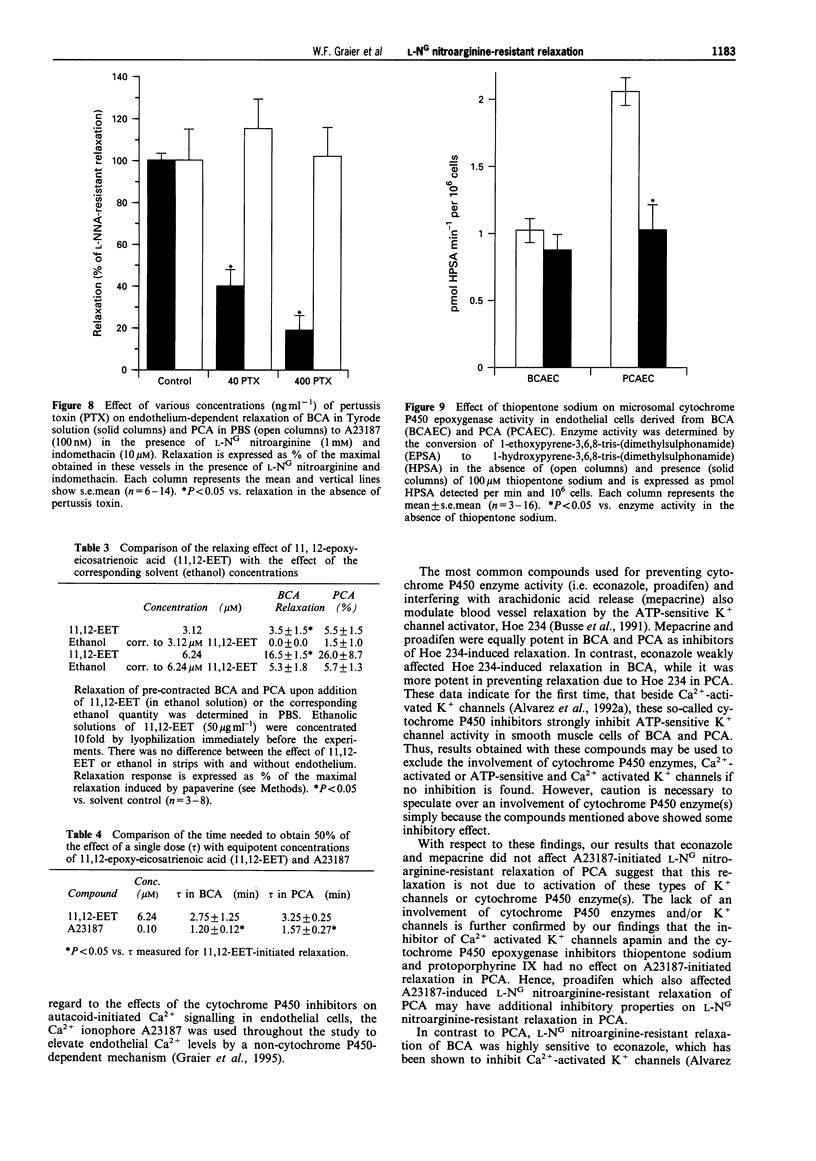
1. Coronary arteries from bovines (BCA) and pigs (PCA) were used for measuring endothelium-dependent relaxation in the presence of L-NG nitroarginine and indomethacin. As some compounds tested have been found to have an inhibitory effect on autacoid-activated endothelial Ca2+ signalling, endothelium-dependent relaxation was initiated with the Ca2+ ionophore A23187. 2. The common compounds for modulating arachidonic acid release/pathway, mepacrine and econazole only inhibited L-NG nitroarginine-resistant relaxation in BCA not in PCA. In contrast, proadifen (SKF 525A) diminished relaxation in BCA and PCA. Mepacrine and proadifen inhibited Hoe-234-initiated relaxation in BCA and PCA, while econazole only inhibited Hoe 234-induced relaxation in PCA. Due to the multiple effects of these compounds, caution is necessary in the interpretation of results obtained with these compounds. 3. The inhibitor of Ca(2+)-activated K+ channels, apamin, strongly attenuated A23187-induced L-NG nitroarginine-resistant relaxation in BCA while apamin did not affect L-NG nitroarginine-resistant relaxation in PCA. 4. Pertussis toxin blunted L-NG nitroarginine-resistant relaxation in BCA, while relaxation of PCA was not affected by pertussis toxin. 5. Thiopentone sodium inhibited endothelial cytochrome P450 epoxygenase (EPO) in PCA but not in BCA, while L-NG nitroarginine-resistant relaxation of BCA and PCA were unchanged. Protoporphyrine IX inhibited EPO in BCA and PCA and abolished L-NG nitroarginine-resistant relaxation of BCA not PCA. 6. An EPO-derived compound, 11,12-epoxy-eicosatrienoic acid (11,12-EET) yielded significant relaxation in BCA and PCA in three out of six experiments. 7. These findings suggest that L-NG nitroarginine-resistant relaxation in BCA and PCA constitutes two distinct pathways. In BCA, activation of Ca(2+)-activated K+ channels via a pertussis-toxin-sensitive G protein and EPO-derived compounds might be involved. In PCA, no selective inhibition of L-NG nitroarginine-resistant relaxation was found.
Full text
PDF



Selected References
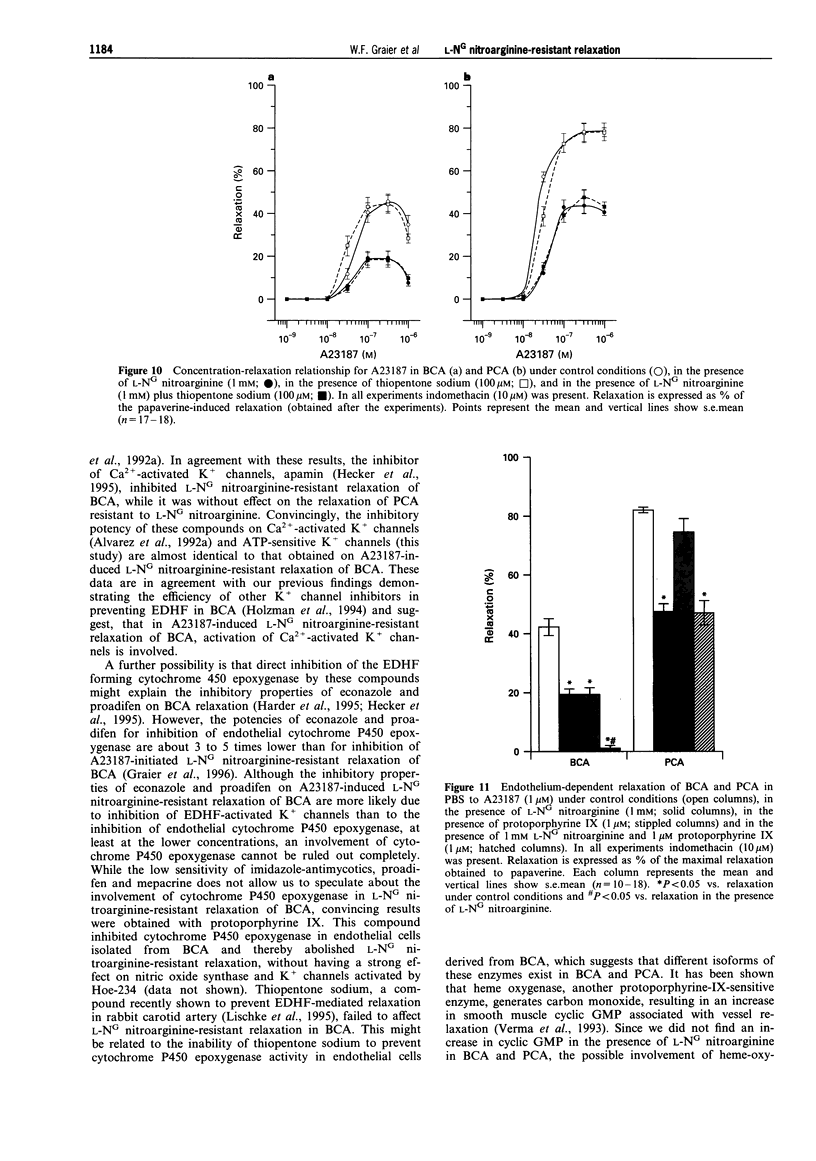
These references are in PubMed. This may not be the complete list of references from this article.
- Adeagbo A. S., Malik K. U. Contribution of K+ channels to arachidonic acid-induced endothelium-dependent vasodilation in rat isolated perfused mesenteric arteries. J Pharmacol Exp Ther. 1991 Aug;258(2):452–458. [PubMed] [Google Scholar]
- Alvarez J., Montero M., Garcia-Sancho J. Cytochrome P450 may regulate plasma membrane Ca2+ permeability according to the filling state of the intracellular Ca2+ stores. FASEB J. 1992 Jan 6;6(2):786–792. doi: 10.1096/fasebj.6.2.1537469. [DOI] [PubMed] [Google Scholar]
- Alvarez J., Montero M., Garcia-Sancho J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J Biol Chem. 1992 Jun 15;267(17):11789–11793. [PubMed] [Google Scholar]
- Alvarez J., Montero M., García-Sancho J. Cytochrome P-450 may link intracellular Ca2+ stores with plasma membrane Ca2+ influx. Biochem J. 1991 Feb 15;274(Pt 1):193–197. doi: 10.1042/bj2740193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Lückhoff A., Mülsch A. Cellular mechanisms controlling EDRF/NO formation in endothelial cells. Basic Res Cardiol. 1991;86 (Suppl 2):7–16. doi: 10.1007/978-3-642-72461-9_2. [DOI] [PubMed] [Google Scholar]
- Cannon S. D., Wilson S. P., Walsh K. B. A G protein-activated K+ current in bovine adrenal chromaffin cells: possible regulatory role in exocytosis. Mol Pharmacol. 1994 Jan;45(1):109–116. [PubMed] [Google Scholar]
- Chen G. F., Cheung D. W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992 Feb;70(2):257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Cole W. C., Carl A., Sanders K. M. Muscarinic suppression of Ca2+-dependent K current in colonic smooth muscle. Am J Physiol. 1989 Sep;257(3 Pt 1):C481–C487. doi: 10.1152/ajpcell.1989.257.3.C481. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D., Ma Y. H., Falck J. R., Roman R. J., VanRollins M., Harder D. R. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992 Aug;263(2 Pt 2):H519–H525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- Graier W. F., Groschner K., Schmidt K., Kukovetz W. R. SK&F 96365 inhibits histamine-induced formation of endothelium-derived relaxing factor in human endothelial cells. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1539–1545. doi: 10.1016/s0006-291x(05)81582-7. [DOI] [PubMed] [Google Scholar]
- Graier W. F., Simecek S., Sturek M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J Physiol. 1995 Jan 15;482(Pt 2):259–274. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada E., Nakajima T., Ota S., Terano A., Omata M., Nakade S., Mikoshiba K., Kurachi Y. Activation of Ca(2+)-dependent K+ current by acetylcholine and histamine in a human gastric epithelial cell line. J Gen Physiol. 1993 Oct;102(4):667–692. doi: 10.1085/jgp.102.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R., Campbell W. B., Roman R. J. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res. 1995 Mar-Apr;32(2):79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- Hecker M., Bara A. T., Bauersachs J., Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 1994 Dec 1;481(Pt 2):407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffner U., Feletou M., Flavahan N. A., Vanhoutte P. M. Canine arteries release two different endothelium-derived relaxing factors. Am J Physiol. 1989 Jul;257(1 Pt 2):H330–H333. doi: 10.1152/ajpheart.1989.257.1.H330. [DOI] [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Holzmann S., Kukovetz W. R., Schmidt K. Mode of action of coronary arterial relaxation by prostacyclin. J Cyclic Nucleotide Res. 1980;6(6):451–460. [PubMed] [Google Scholar]
- Holzmann S., Kukovetz W. R., Windischhofer W., Paschke E., Graier W. F. Pharmacologic differentiation between endothelium-dependent relaxations sensitive and resistant to nitro-L-arginine in coronary arteries. J Cardiovasc Pharmacol. 1994 May;23(5):747–756. doi: 10.1097/00005344-199405000-00009. [DOI] [PubMed] [Google Scholar]
- Hu S., Kim H. S. Activation of K+ channel in vascular smooth muscles by cytochrome P450 metabolites of arachidonic acid. Eur J Pharmacol. 1993 Jan 12;230(2):215–221. doi: 10.1016/0014-2999(93)90805-r. [DOI] [PubMed] [Google Scholar]
- Kume H., Mikawa K., Takagi K., Kotlikoff M. I. Role of G proteins and KCa channels in the muscarinic and beta-adrenergic regulation of airway smooth muscle. Am J Physiol. 1995 Feb;268(2 Pt 1):L221–L229. doi: 10.1152/ajplung.1995.268.2.L221. [DOI] [PubMed] [Google Scholar]
- Lischke V., Busse R., Hecker M. Selective inhibition by barbiturates of the synthesis of endothelium-derived hyperpolarizing factor in the rabbit carotid artery. Br J Pharmacol. 1995 Jul;115(6):969–974. doi: 10.1111/j.1476-5381.1995.tb15905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher T. F., Noll G. Endothelium dysfunction in the coronary circulation. J Cardiovasc Pharmacol. 1994;24 (Suppl 3):S16–S26. [PubMed] [Google Scholar]
- Mason M. J., Mayer B., Hymel L. J. Inhibition of Ca2+ transport pathways in thymic lymphocytes by econazole, miconazole, and SKF 96365. Am J Physiol. 1993 Mar;264(3 Pt 1):C654–C662. doi: 10.1152/ajpcell.1993.264.3.C654. [DOI] [PubMed] [Google Scholar]
- Montero M., Alvarez J., Garcia-Sancho J. Agonist-induced Ca2+ influx in human neutrophils is secondary to the emptying of intracellular calcium stores. Biochem J. 1991 Jul 1;277(Pt 1):73–79. doi: 10.1042/bj2770073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M., Alvarez J., García-Sancho J. Control of plasma-membrane Ca2+ entry by the intracellular Ca2+ stores. Kinetic evidence for a short-lived mediator. Biochem J. 1992 Dec 1;288(Pt 2):519–525. doi: 10.1042/bj2880519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M., Mombouli J. V., Taylor A. A., Vanhoutte P. M. Endothelium-dependent hyperpolarization caused by bradykinin in human coronary arteries. J Clin Invest. 1993 Dec;92(6):2867–2871. doi: 10.1172/JCI116907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., MacLeod K. M. Effect of carbachol in the absence and presence of phenylephrine on Rb+ efflux and tension in rabbit left atria. Eur J Pharmacol. 1994 May 2;256(3):311–319. doi: 10.1016/0014-2999(94)90557-6. [DOI] [PubMed] [Google Scholar]
- Sargeant P., Clarkson W. D., Sage S. O., Heemskerk J. W. Calcium influx evoked by Ca2+ store depletion in human platelets is more susceptible to cytochrome P-450 inhibitors than receptor-mediated calcium entry. Cell Calcium. 1992 Oct;13(9):553–564. doi: 10.1016/0143-4160(92)90035-q. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Chen G., Yamamoto Y. Endothelium-derived hyperpolarizing factor (EDHF). Jpn Circ J. 1992 Feb;56(2):170–174. doi: 10.1253/jcj.56.170. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Takahashi K., Sakai O. Regulation by GTP of a Ca(2+)-activated K+ channel in the apical membrane of rabbit cortical collecting duct cells. J Membr Biol. 1994 Jul;141(1):43–50. doi: 10.1007/BF00232872. [DOI] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]


