Abstract
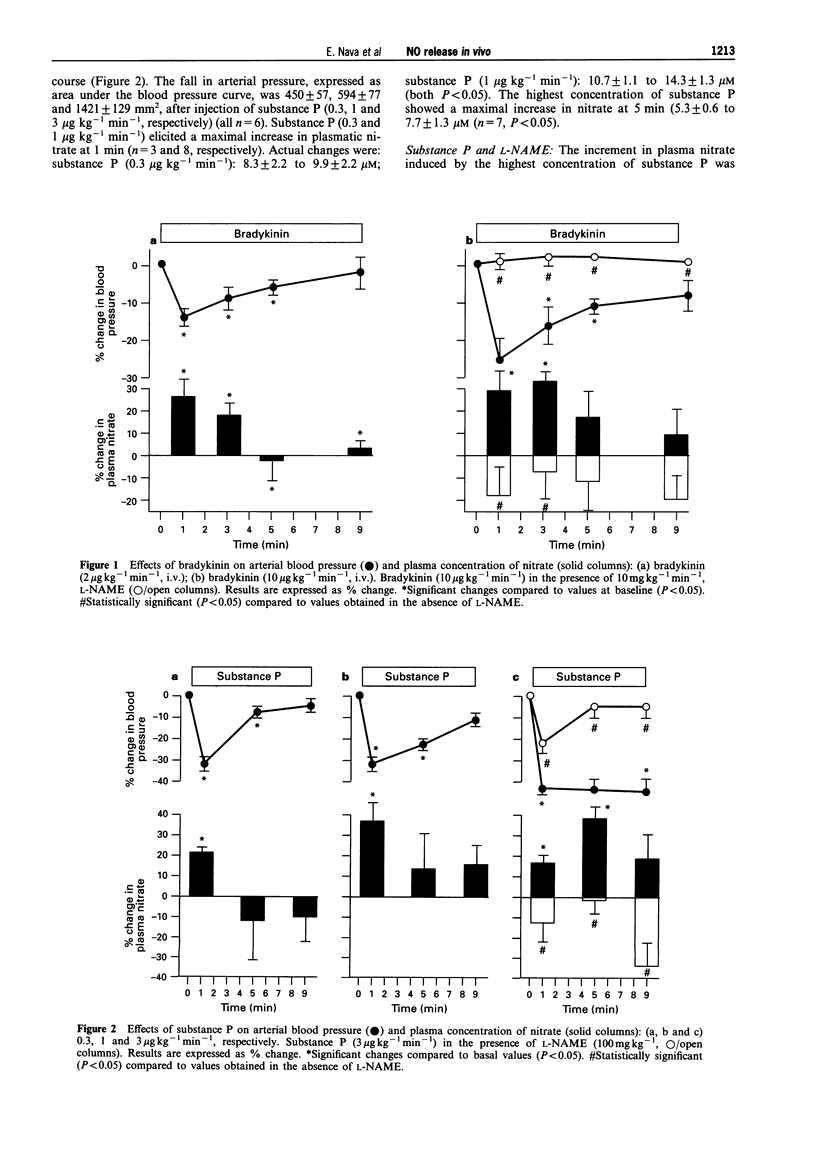
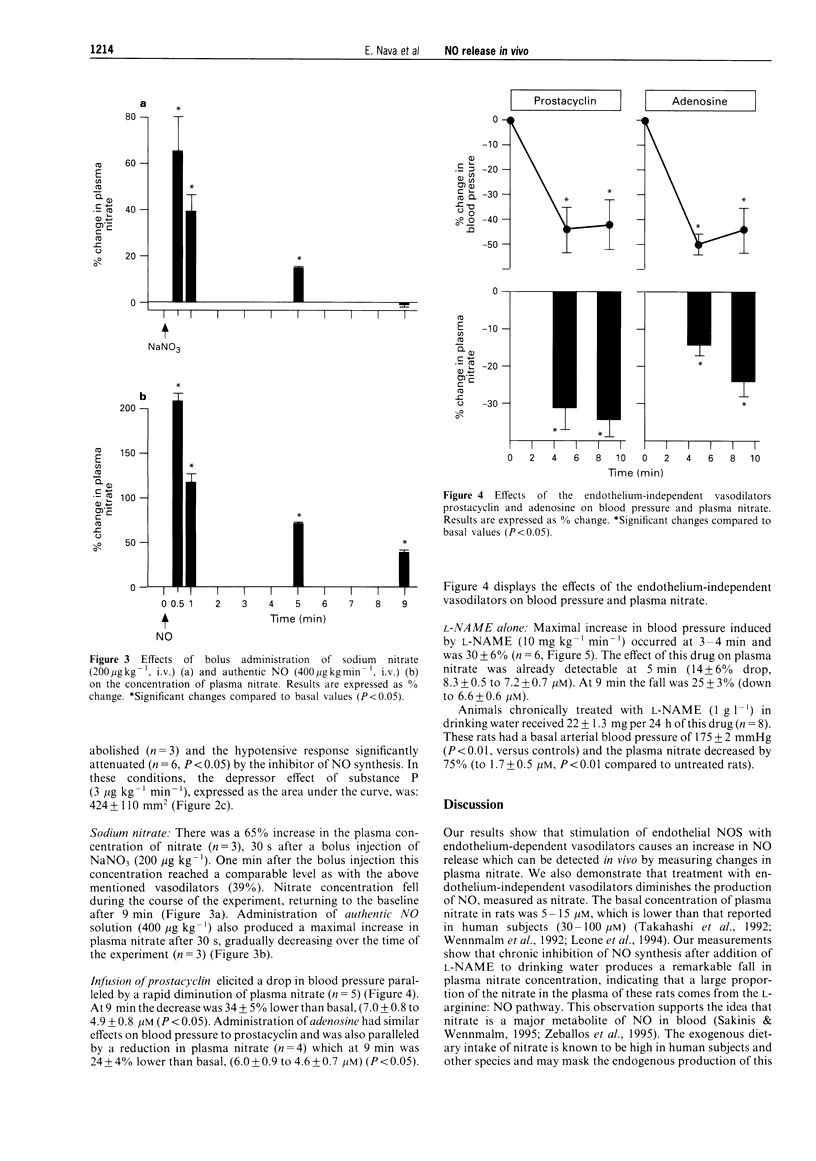
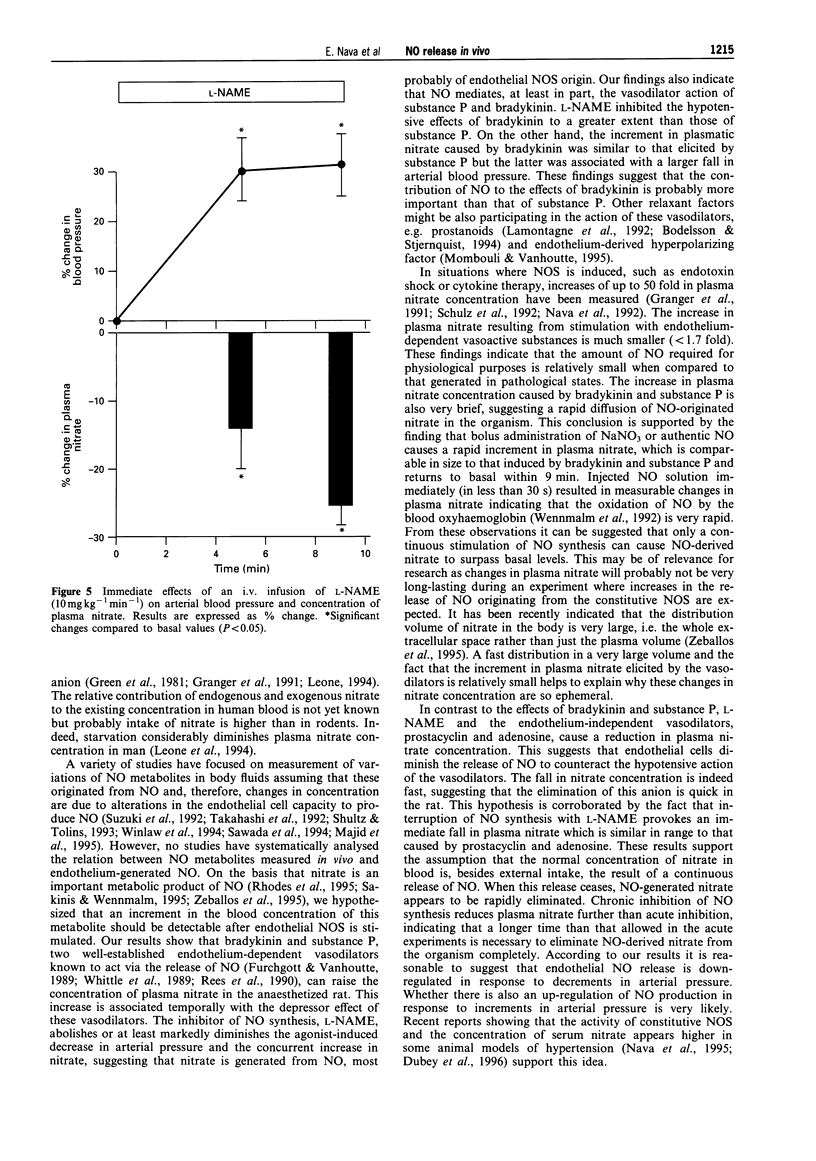
1. Changes in the release of nitric oxide (NO) in vivo were studied in rats following the administration of endothelium-dependent and -independent vasodilators as well as the NO synthesis inhibitor, NG-nitro-L-arginine methyl ester (L-NAME). NO production was assessed by measuring variations of nitrate in plasma by capillary ion analysis. 2. Intravenous administration of the endothelium-dependent vasodilators, bradykinin (2 and 10 micrograms kg-1 min-1) or substance P (0.3-3 micrograms kg-1 min-1) caused a transient dose-dependent hypotension followed by an increase in plasma nitrate concentration (maximal increments: 33 +/- 5% and 38 +/- 6%, for bradykinin and substance P, respectively). Prior administration of L-NAME (10 mg kg-1 min-1) inhibited the hypotension and increase in plasma nitrate caused by these substances. Intravenous administration of sodium nitrate (200 micrograms kg-1) also produced a transitory elevation in plasma nitrate which was similar in magnitude as that caused by the vasodilators. A rapid and transitory increment in plasma nitrate was observed after i.v. administration of authentic NO (400 micrograms kg-1). 3. Rats receiving the endothelium-dependent vasodilators, prostacyclin (0.6 micrograms kg-1 min-1) or adenosine (3 mg kg-1 min-1) intravenously showed a drop in blood pressure paralleled by a decrease in plasma nitrate (maximal decreases: 34 +/- 5% and 24 +/- 4%, for prostacyclin and adenosine, respectively). A similar effect on the plasmatic concentration of nitrate was observed when L-NAME (10 mg kg-1 min-1, i.v.) was administered to the animals. 4. This study demonstrates that (i) changes in plasma nitrate can be detected in vivo after stimulation or inhibition of NO synthase, (ii) an increased production of NO, measured as plasma nitrate, is related to the hypotension caused by bradykinin and substance P and (iii) a diminished concentration of plasmatic nitrate is associated to the hypotension induced by adenosine or prostacyclin (endothelium-independent vasodilators), suggesting that the L-arginine: NO pathway is capable of rapid down-regulation in response to a fall in blood pressure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodelsson G., Stjernquist M. Endothelium-dependent relaxation to substance P in human umbilical artery is mediated via prostanoid synthesis. Hum Reprod. 1994 Apr;9(4):733–737. doi: 10.1093/oxfordjournals.humrep.a138580. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Broadnax L. M. Urinary nitrate excretion in relation to murine macrophage activation. Influence of dietary L-arginine and oral NG-monomethyl-L-arginine. J Immunol. 1991 Feb 15;146(4):1294–1302. [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne D., König A., Bassenge E., Busse R. Prostacyclin and nitric oxide contribute to the vasodilator action of acetylcholine and bradykinin in the intact rabbit coronary bed. J Cardiovasc Pharmacol. 1992 Oct;20(4):652–657. doi: 10.1097/00005344-199210000-00020. [DOI] [PubMed] [Google Scholar]
- Leone A. M., Francis P. L., Rhodes P., Moncada S. A rapid and simple method for the measurement of nitrite and nitrate in plasma by high performance capillary electrophoresis. Biochem Biophys Res Commun. 1994 Apr 29;200(2):951–957. doi: 10.1006/bbrc.1994.1542. [DOI] [PubMed] [Google Scholar]
- Majid D. S., Godfrey M., Grisham M. B., Navar L. G. Relation between pressure natriuresis and urinary excretion of nitrate/nitrite in anesthetized dogs. Hypertension. 1995 Apr;25(4 Pt 2):860–865. doi: 10.1161/01.hyp.25.4.860. [DOI] [PubMed] [Google Scholar]
- Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992 Jul;145(3):201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Nava E., Noll G., Lüscher T. F. Increased activity of constitutive nitric oxide synthase in cardiac endothelium in spontaneous hypertension. Circulation. 1995 May 1;91(9):2310–2313. doi: 10.1161/01.cir.91.9.2310. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes P., Leone A. M., Francis P. L., Struthers A. D., Moncada S., Rhodes PM [corrected to Rhodes P. ]. The L-arginine:nitric oxide pathway is the major source of plasma nitrite in fasted humans. Biochem Biophys Res Commun. 1995 Apr 17;209(2):590–596. doi: 10.1006/bbrc.1995.1541. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Sakamaki T., Nakamura T., Sato K., Ono Z., Murata K. Release of nitric oxide in response to acetylcholine is unaltered in spontaneously hypertensive rats. J Hypertens. 1994 Jul;12(7):745–750. [PubMed] [Google Scholar]
- Schulz R., Nava E., Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992 Mar;105(3):575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz P. J., Tolins J. P. Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest. 1993 Feb;91(2):642–650. doi: 10.1172/JCI116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. C., Jr AHA president's letter. Circulation. 1995 Jul 1;92(1):1–1. [PubMed] [Google Scholar]
- Suzuki H., Ikenaga H., Hishikawa K., Nakaki T., Kato R., Saruta T. Increases in NO2-/NO3- excretion in the urine as an indicator of the release of endothelium-derived relaxing factor during elevation of blood pressure. Clin Sci (Lond) 1992 Jun;82(6):631–634. doi: 10.1042/cs0820631. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Nakanishi T., Nishimura M., Tanaka H., Yoshimura M. Measurements of serum levels of nitrate ions in men and women: implications of endothelium-derived relaxing factor in blood pressure regulation and atherosclerosis. J Cardiovasc Pharmacol. 1992;20 (Suppl 12):S214–S216. doi: 10.1097/00005344-199204002-00061. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Wennmalm A., Benthin G., Petersson A. S. Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. Br J Pharmacol. 1992 Jul;106(3):507–508. doi: 10.1111/j.1476-5381.1992.tb14365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winlaw D. S., Smythe G. A., Keogh A. M., Schyvens C. G., Spratt P. M., Macdonald P. S. Increased nitric oxide production in heart failure. Lancet. 1994 Aug 6;344(8919):373–374. doi: 10.1016/s0140-6736(94)91403-6. [DOI] [PubMed] [Google Scholar]
- Zeballos G. A., Bernstein R. D., Thompson C. I., Forfia P. R., Seyedi N., Shen W., Kaminiski P. M., Wolin M. S., Hintze T. H. Pharmacodynamics of plasma nitrate/nitrite as an indication of nitric oxide formation in conscious dogs. Circulation. 1995 Jun 15;91(12):2982–2988. doi: 10.1161/01.cir.91.12.2982. [DOI] [PubMed] [Google Scholar]


