Abstract
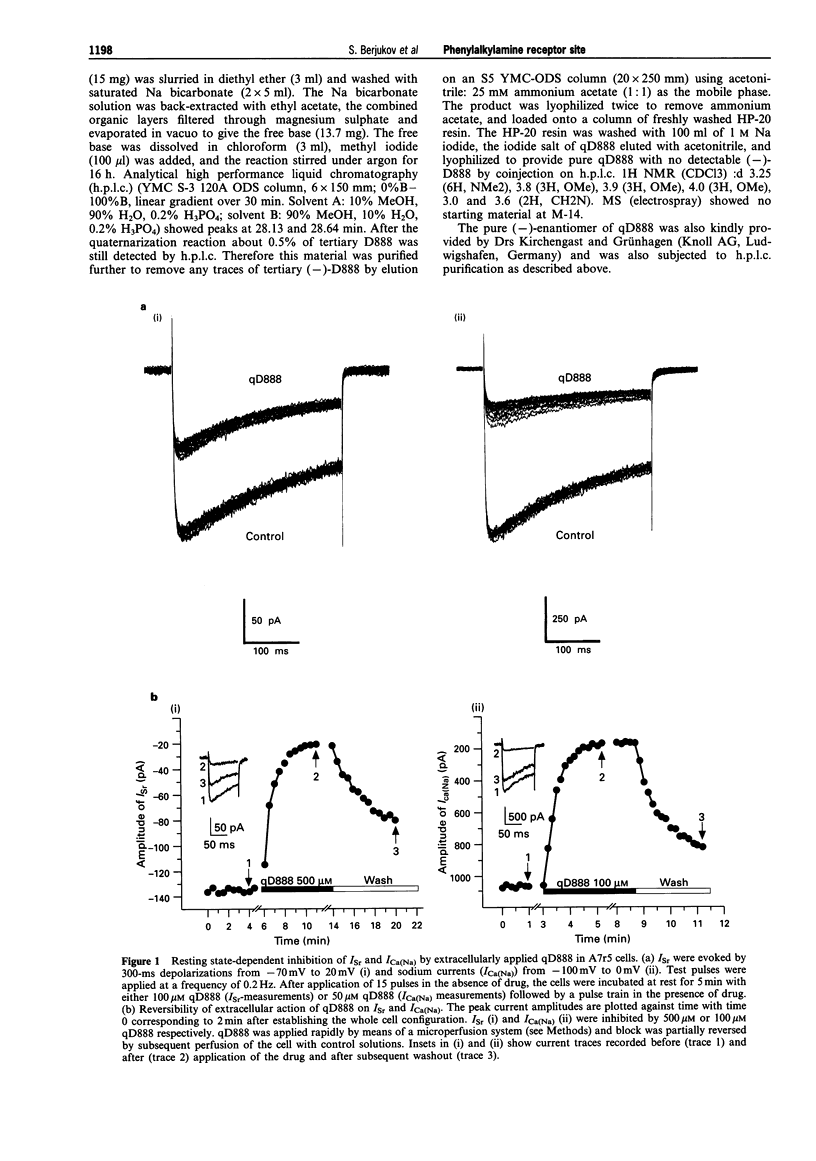
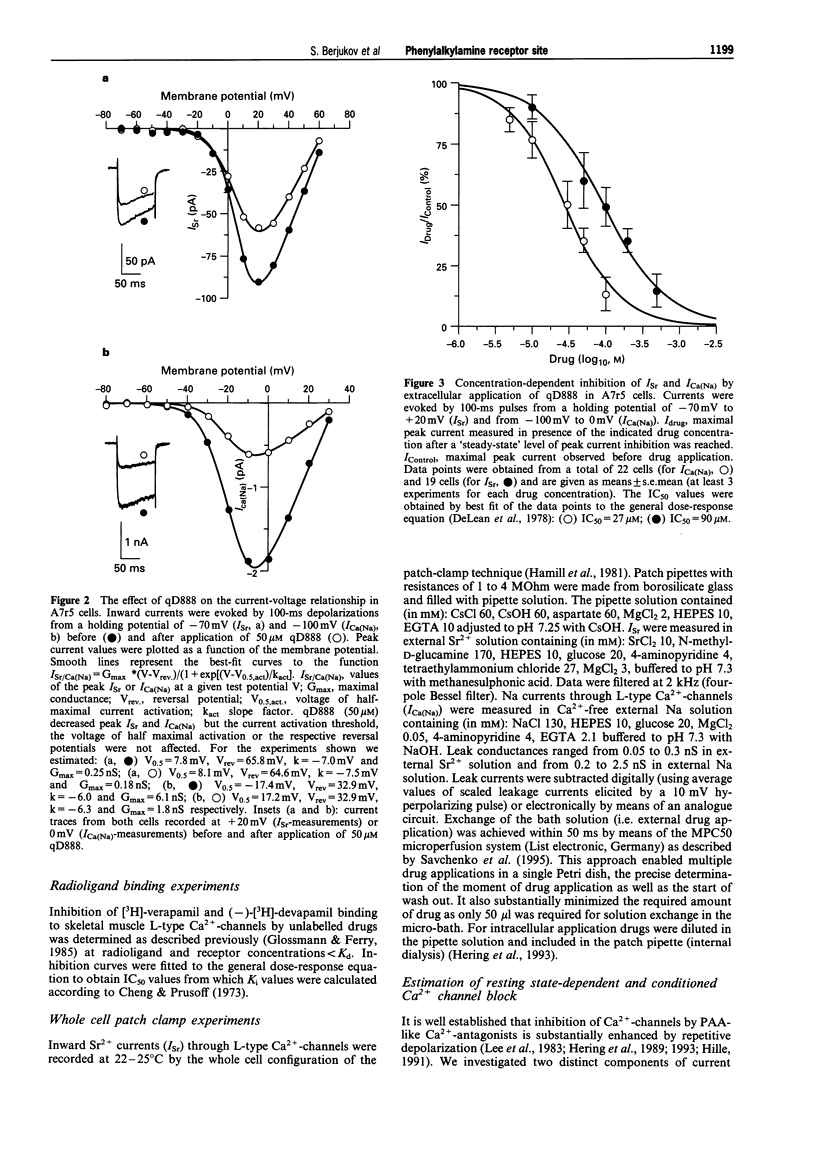
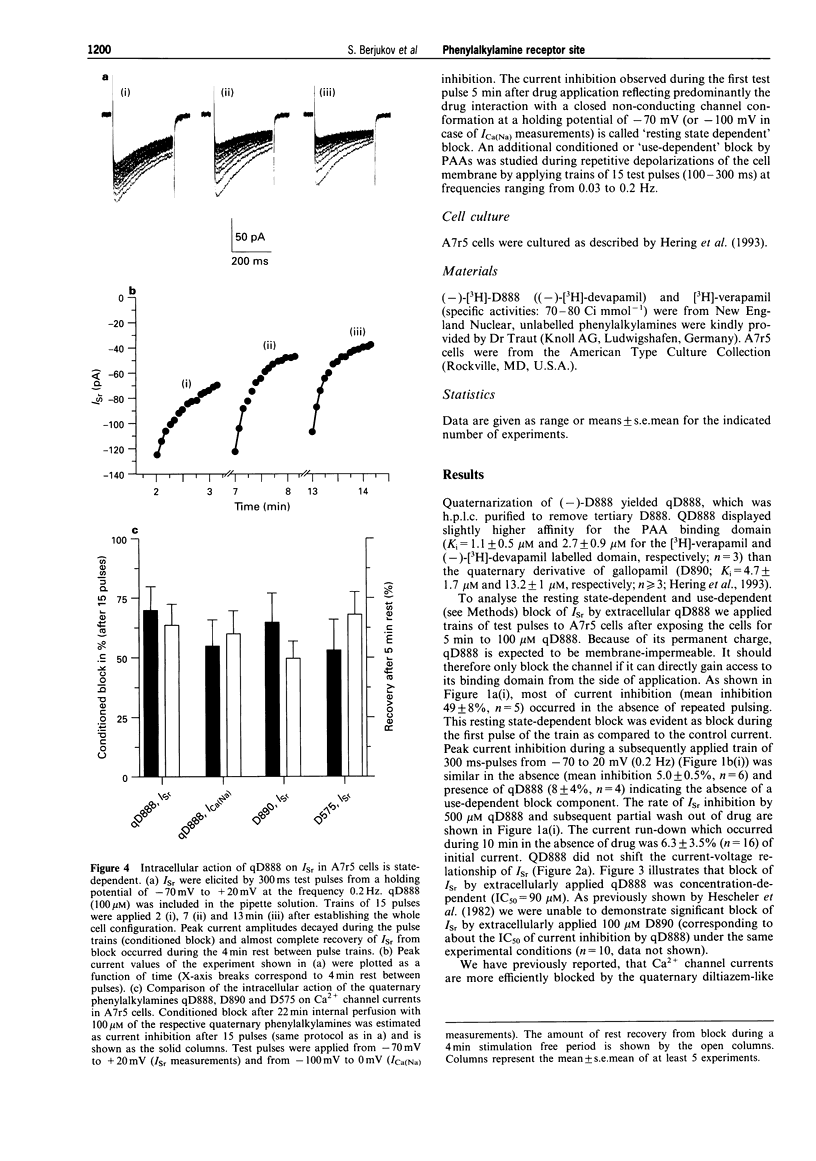
1. The quaternary derivative of the potent verapamil-analogue, (-)-D888, (qD888, 4-cyano-4-(3,4-dimethoxyphenyl)-N-[2-(3-methoxy phenyl)ethyl]-N,N,5-trimethyl-1-hexanaminium) was synthesized as a novel membrane-impermeable probe to study the localization of phenylalkylamine (PAA) effector domains on L-type Ca2+ channels. Channel block by qD888 was investigated in smooth muscle-like (A7r5) cells after extra- and intracellular application by use of the whole-cell configuration of the patch clamp technique. 2. Extracellularly applied qD888 inhibited Sr2+ (Isr) (IC50 = 90 microM) and Na+ (IC50 = 27 microM) inward currents through L-type Ca(2+)-channels mainly in a resting-state-dependent manner. Structurally closely related quaternary PAAs (e.g. D890) were ineffective after extracellular application. 3. QD888 also blocked Isr from the cytoplasmic side, as did other quaternary PAAs (D890, D575). Intracellular block was clearly dependent on channel opening, which resulted in pronounced use-dependence. 4. We conclude that qD888 blocks L-type Ca2+ channels not only from the intracellular side, via interaction with the classical PAA binding domain, but also from the extracellular channel surface. The properties of Ca2+ channel block together with previous biochemical and structural data suggest that extracellular block may be mediated by a site that also confers tonic block by quaternary benzothiazepines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi-Akahane S., Amano Y., Okuyama R., Nagao T. Quaternary diltiazem can act from both sides of the membrane in ventricular myocytes. Jpn J Pharmacol. 1993 Mar;61(3):263–266. doi: 10.1254/jjp.61.263. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Striessnig J. Receptor sites for Ca2+ channel antagonists. Trends Pharmacol Sci. 1992 Jun;13(6):256–262. doi: 10.1016/0165-6147(92)90079-l. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Döring F., Degtiar V. E., Grabner M., Striessnig J., Hering S., Glossman H. Transfer of L-type calcium channel IVS6 segment increases phenylalkylamine sensitivity of alpha1A. J Biol Chem. 1996 May 17;271(20):11745–11749. doi: 10.1074/jbc.271.20.11745. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R. Assay for calcium channels. Methods Enzymol. 1985;109:513–550. doi: 10.1016/0076-6879(85)09112-1. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Goll A., Ferry D. R., Glossmann H. Target size analysis and molecular properties of Ca2+ channels labelled with [3H]verapamil. Eur J Biochem. 1984 May 15;141(1):177–186. doi: 10.1111/j.1432-1033.1984.tb08172.x. [DOI] [PubMed] [Google Scholar]
- Goll A., Ferry D. R., Striessnig J., Schober M., Glossmann H. (-)-[3H]Desmethoxyverapamil, a novel Ca2+ channel probe. Binding characteristics and target size analysis of its receptor in skeletal muscle. FEBS Lett. 1984 Oct 29;176(2):371–377. doi: 10.1016/0014-5793(84)81199-0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hering S., Bolton T. B., Beech D. J., Lim S. P. Mechanism of calcium channel block by D600 in single smooth muscle cells from rabbit ear artery. Circ Res. 1989 May;64(5):928–936. doi: 10.1161/01.res.64.5.928. [DOI] [PubMed] [Google Scholar]
- Hering S., Savchenko A., Strübing C., Lakitsch M., Striessnig J. Extracellular localization of the benzothiazepine binding domain of L-type Ca2+ channels. Mol Pharmacol. 1993 May;43(5):820–826. [PubMed] [Google Scholar]
- Hescheler J., Pelzer D., Trube G., Trautwein W. Does the organic calcium channel blocker D600 act from inside or outside on the cardiac cell membrane? Pflugers Arch. 1982 Jun;393(4):287–291. doi: 10.1007/BF00581411. [DOI] [PubMed] [Google Scholar]
- Hockerman G. H., Johnson B. D., Scheuer T., Catterall W. A. Molecular determinants of high affinity phenylalkylamine block of L-type calcium channels. J Biol Chem. 1995 Sep 22;270(38):22119–22122. doi: 10.1074/jbc.270.38.22119. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Arena J. P., Chin S. Block of L-type calcium channels by charged dihydropyridines. Sensitivity to side of application and calcium. J Gen Physiol. 1991 Jul;98(1):63–75. doi: 10.1085/jgp.98.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S. D., Floyd D. M., Das J., Hunt J. T., Krapcho J., Rovnyak G., Duff K. J., Lee V. G., Moquin R. V., Turk C. F. Benzazepinone calcium channel blockers. 4. Structure-activity overview and intracellular binding site. J Med Chem. 1992 Feb 21;35(4):780–793. doi: 10.1021/jm00082a020. [DOI] [PubMed] [Google Scholar]
- Kimball S. D., Hunt J. T., Barrish J. C., Das J., Floyd D. M., Lago M. W., Lee V. G., Spergel S. H., Moreland S., Hedberg S. A. 1-Benzazepin-2-one calcium channel blockers--VI. Receptor-binding model and possible relationship to desmethoxyverapamil. Bioorg Med Chem. 1993 Oct;1(4):285–307. doi: 10.1016/s0968-0896(00)82134-3. [DOI] [PubMed] [Google Scholar]
- Lacinová L., Ludwig A., Bosse E., Flockerzi V., Hofmann F. The block of the expressed L-type calcium channel is modulated by the beta 3 subunit. FEBS Lett. 1995 Oct 9;373(2):103–107. doi: 10.1016/0014-5793(95)01013-5. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Voltage-sensitive Ca2+ channels. J Biol Chem. 1992 Jan 25;267(3):1403–1406. [PubMed] [Google Scholar]
- Reynolds I. J., Snowman A. M., Snyder S. H. (-)-[3H] desmethoxyverapamil labels multiple calcium channel modulator receptors in brain and skeletal muscle membranes: differentiation by temperature and dihydropyridines. J Pharmacol Exp Ther. 1986 Jun;237(3):731–738. [PubMed] [Google Scholar]
- Savchenko A., Glossmann H., Hering S. Improved micro-perfusion chamber for multiple and rapid solution exchange in adherent single cells. Pflugers Arch. 1995 Jan;429(3):436–442. doi: 10.1007/BF00374161. [DOI] [PubMed] [Google Scholar]
- Seydl K., Kimball D., Schindler H., Romanin C. The benzazepine/benzothiazepine binding domain of the cardiac L-type Ca2+ channel is accessible only from the extracellular side. Pflugers Arch. 1993 Sep;424(5-6):552–554. doi: 10.1007/BF00374922. [DOI] [PubMed] [Google Scholar]
- Striessnig J., Goll A., Moosburger K., Glossmann H. Purified calcium channels have three allosterically coupled drug receptors. FEBS Lett. 1986 Mar 3;197(1-2):204–210. doi: 10.1016/0014-5793(86)80327-1. [DOI] [PubMed] [Google Scholar]
- Striessnig J., Knaus H. G., Grabner M., Moosburger K., Seitz W., Lietz H., Glossmann H. Photoaffinity labelling of the phenylalkylamine receptor of the skeletal muscle transverse-tubule calcium channel. FEBS Lett. 1987 Feb 23;212(2):247–253. doi: 10.1016/0014-5793(87)81354-6. [DOI] [PubMed] [Google Scholar]
- Wegener J. W., Nawrath H. Extracellular site of action of phenylalkylamines on L-type calcium current in rat ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1995 Sep;352(3):322–330. doi: 10.1007/BF00168564. [DOI] [PubMed] [Google Scholar]
- White E. J., Bradford H. F. Participation of intracellular sites in the action of Ca2+ channel blockers. Eur J Pharmacol. 1986 Nov 4;130(3):243–248. doi: 10.1016/0014-2999(86)90274-8. [DOI] [PubMed] [Google Scholar]


