Abstract
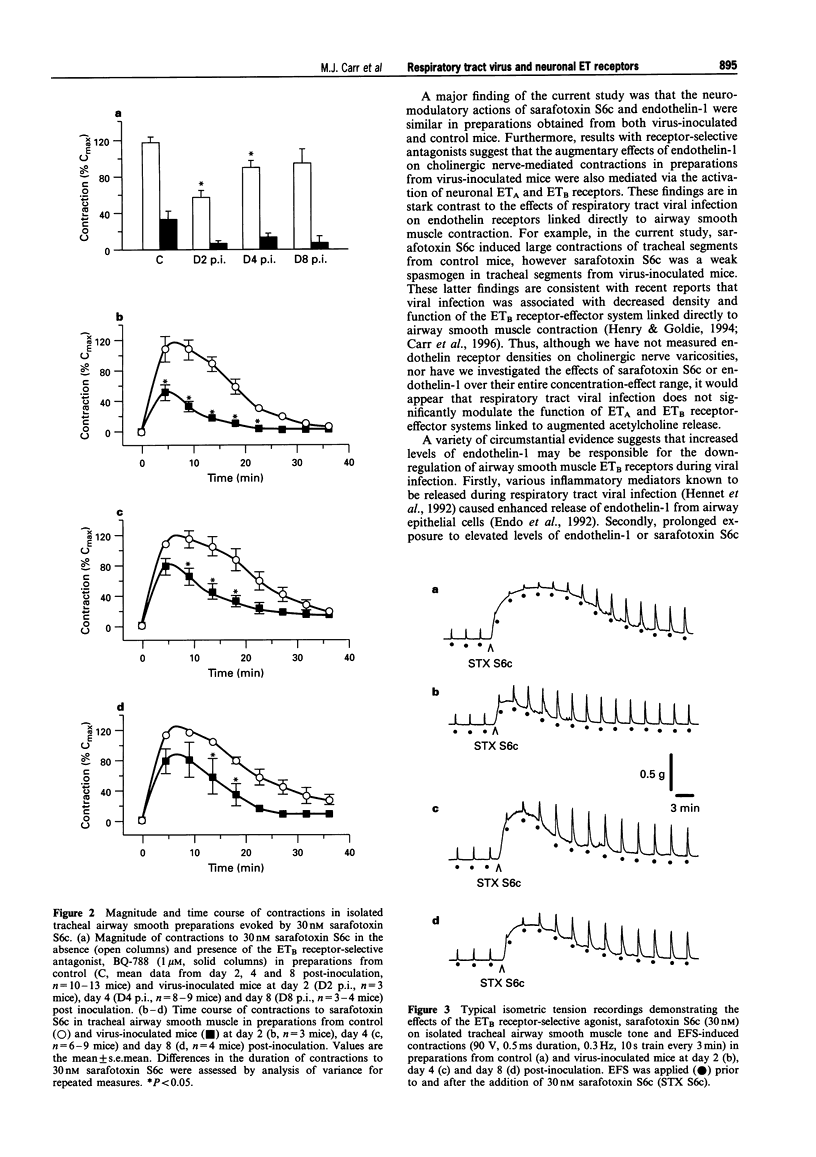
1. This study examined the influence of respiratory tract infection with influenza A/PR-8/34 virus on endothelin receptor-mediated modulation of contraction induced by stimulation of cholinergic nerves in mouse isolated trachea. 2. The ETB receptor-selective agonist, sarafotoxin S6c (30 nM) induced large transient contractions (118 +/- 5% Cmax, n = 13; where Cmax is the contraction induced by 10 microM carbachol) of isolated tracheal segments from control mice. The peak contractile response to 30 nM sarafotoxin S6c was significantly lower in preparations from virus-inoculated mice at day 2 (57 +/- 8% Cmax, n = 3, P < 0.05) and 4 post-inoculation (90 +/- 8% Cmax, n = 9, P < 0.05), consistent with virus-induced attentuation of the ETB receptor-effector system linked to airway smooth muscle contraction. The mean peak contraction to 30 nM sarafotoxin S6c of preparations from virus-inoculated mice at day 8 post-inoculation (94 +/- 17% Cmax, n = 4) was not significantly different from that of control. 3. Electrical field stimulation (EFS; 90 V, 0.5 ms duration, 10 s train, 0.1-30 Hz) of preparations from control and virus-inoculated mice, caused contractions that were abolished by 0.1 microM atropine or 3 microM tetrodotoxin, indicating that these responses were mediated by neuronally released acetylcholine. Sarafotoxin S6c markedly potentiated contractions induced by a standard stimulus (0.3 Hz, every 3 min) in tracheal segments from control and virus-inoculated mice. In tracheal tissue from control mice, 30 nM sarafotoxin S6c significantly increased a standard EFS-induced contraction of 24 +/- 4% Cmax by a further 24 +/- 3% Cmax (i.e. 2 fold increase, n = 11). Sarafotoxin S6c (30 nM) also markedly potentiated standard EFS-induced contractions in preparations from virus-inoculated mice at day 2 (17 +/- 2% Cmax, n = 3), day 4 (17 +/- 5% Cmax, n = 9) and day 8 (26 +/- 5% Cmax, n = 4) post-inoculation. The level of potentiation of EFS-induced contractions in preparations from virus-inoculated mice was similar to that in tissue from control mice at days, 2, 4 and 8 post-inoculation. In contrast, sarafotoxin S6c (30 nM) did not enhance contractile responses of tracheal segments from control and virus-inoculated mice to exogenously applied acetylcholine (n = 3). 4. Endothelin-1 (1 nM) caused similar potentiations of standard EFS-induced contractions in tracheal segments from control (13 +/- 2% Cmax, n = 23) and virus-inoculated mice at day 2 (13 +/- 1% Cmax, n = 5), day 4 (16 +/- 5% Cmax, n = 6), and day 8 (13 +/- 3% Cmax, n = 8) post-inoculation. In contrast, 1 nM endothelin-1 did not enhance contractile responses of tracheal segments from control and virus-inoculated mice to exogenously applied acetylcholine (n = 4). Neither the ETA receptor-selective antagonist, BQ-123 (3 microM) nor the ETB receptor-selective antagonist, BQ-788 (1 microM) alone had any significant inhibitory effect on endothelin-1-induced potentiations of tracheal segments from control or virus-inoculated mice at days 2, 4 and 8 post-inoculation. However, simultaneous pre-incubation with BQ-123 (3 microM) and BQ-788 (1 microM) prevented endothelin-1-evoked potentiations, indicative of a role for both ETA and ETB receptors in this system. 5. These data clearly demonstrate that respiratory tract viral infection attenuated the function of the postjunctional ETB receptor-effector system linked directly to airway smooth muscle contraction. However, the function of prejunctional ETA and ETB receptor-effector systems linked to augmentation of cholinergic nerve-mediated airway smooth muscle contraction remained unaffected during respiratory tract viral infection in mice.
Full text
PDF

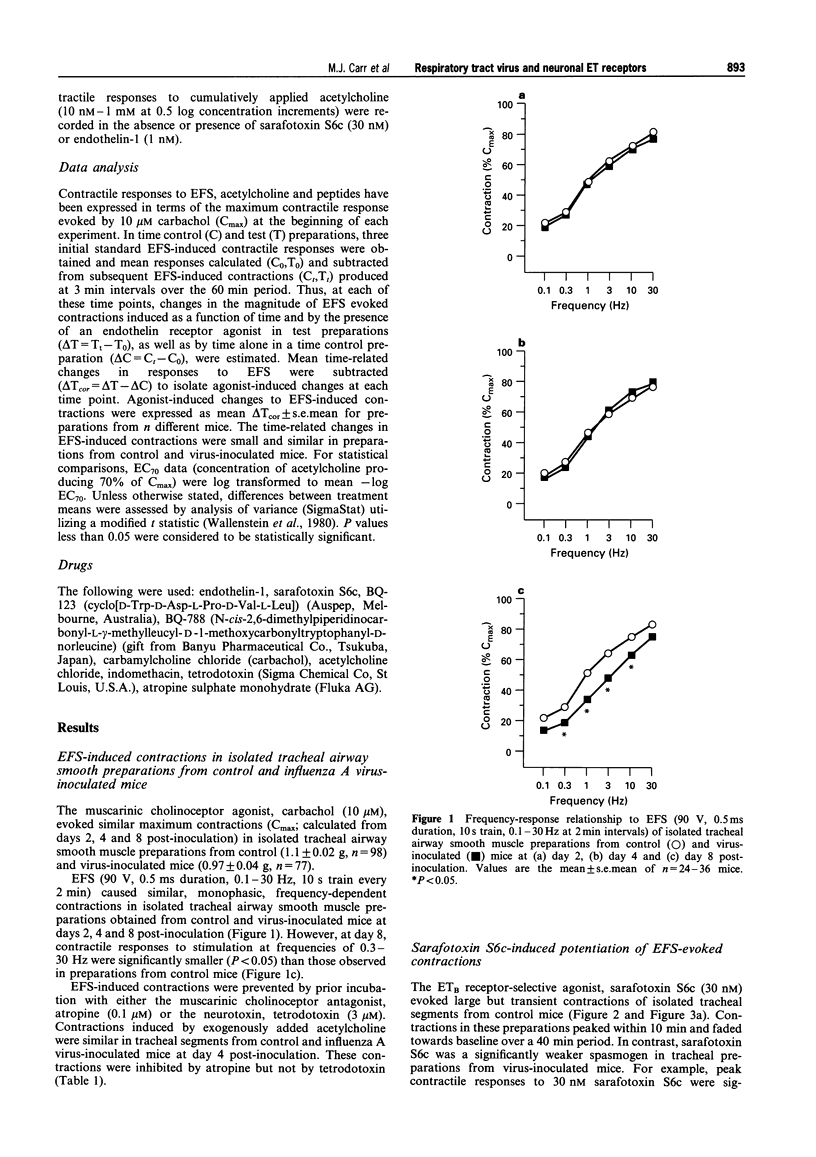

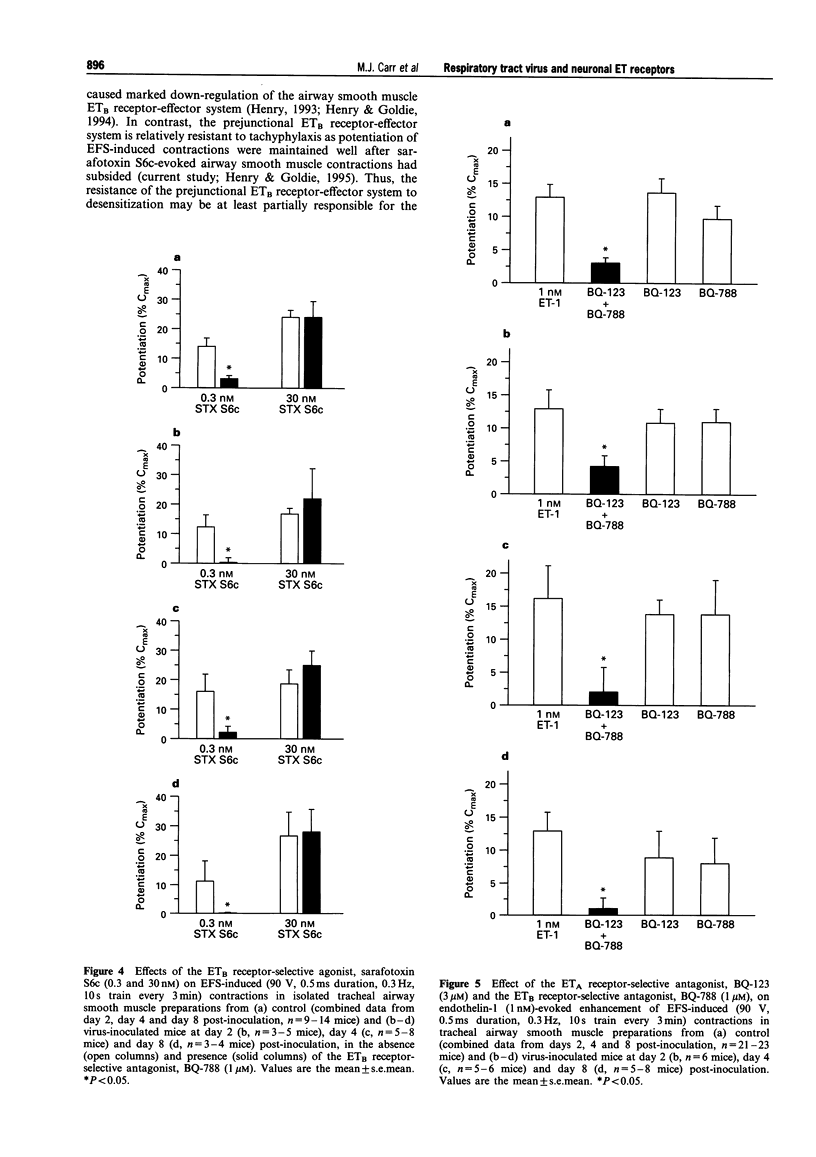


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquilina A. T., Hall W. J., Douglas R. G., Jr, Utell M. J. Airway reactivity in subjects with viral upper respiratory tract infections: the effects of exercise and cold air. Am Rev Respir Dis. 1980 Jul;122(1):3–10. doi: 10.1164/arrd.1980.122.1.3. [DOI] [PubMed] [Google Scholar]
- Beasley R., Coleman E. D., Hermon Y., Holst P. E., O'Donnell T. V., Tobias M. Viral respiratory tract infection and exacerbations of asthma in adult patients. Thorax. 1988 Sep;43(9):679–683. doi: 10.1136/thx.43.9.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. N., Ghatei M. A., Takahashi K., Bretherton-Watt D., Krausz T., Dollery C. T., Bloom S. R. Formation of endothelin by cultured airway epithelial cells. FEBS Lett. 1989 Sep 11;255(1):129–132. doi: 10.1016/0014-5793(89)81075-0. [DOI] [PubMed] [Google Scholar]
- Buckner C. K., Songsiridej V., Dick E. C., Busse W. W. In vivo and in vitro studies on the use of the guinea pig as a model for virus-provoked airway hyperreactivity. Am Rev Respir Dis. 1985 Aug;132(2):305–310. doi: 10.1164/arrd.1985.132.2.305. [DOI] [PubMed] [Google Scholar]
- Carr M. J., Goldie R. G., Henry P. J. Time course of changes in ETB receptor density and function in tracheal airway smooth muscle during respiratory tract viral infection in mice. Br J Pharmacol. 1996 Mar;117(6):1222–1228. doi: 10.1111/j.1476-5381.1996.tb16719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empey D. W., Laitinen L. A., Jacobs L., Gold W. M., Nadel J. A. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976 Feb;113(2):131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- Endo T., Uchida Y., Matsumoto H., Suzuki N., Nomura A., Hirata F., Hasegawa S. Regulation of endothelin-1 synthesis in cultured guinea pig airway epithelial cells by various cytokines. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1594–1599. doi: 10.1016/s0006-291x(05)81590-6. [DOI] [PubMed] [Google Scholar]
- FAZEKAS DE ST GROTH S., WHITE D. O. An improved assay for the infectivity of in influenza viruses. J Hyg (Lond) 1958 Mar;56(1):151–162. doi: 10.1017/s0022172400037621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerts G., Nijkamp F. P. Virus-induced airway hyperresponsiveness. Role of inflammatory cells and mediators. Am J Respir Crit Care Med. 1995 May;151(5):1666–1674. doi: 10.1164/ajrccm.151.5.7735631. [DOI] [PubMed] [Google Scholar]
- Fryer A. D., el-Fakahany E. E., Jacoby D. B. Parainfluenza virus type 1 reduces the affinity of agonists for muscarinic receptors in guinea-pig lung and heart. Eur J Pharmacol. 1990 May 31;181(1-2):51–58. doi: 10.1016/0014-2999(90)90244-z. [DOI] [PubMed] [Google Scholar]
- Garssen J., Van Loveren H., Gierveld C. M., Van der Vliet H., Nijkamp F. P. Functional characterization of muscarinic receptors in murine airways. Br J Pharmacol. 1993 May;109(1):53–60. doi: 10.1111/j.1476-5381.1993.tb13530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie R. G., Grayson P. S., Knott P. G., Self G. J., Henry P. J. Predominance of endothelinA (ETA) receptors in ovine airway smooth muscle and their mediation of ET-1-induced contraction. Br J Pharmacol. 1994 Jul;112(3):749–756. doi: 10.1111/j.1476-5381.1994.tb13142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie R. G., Henry P. J., Knott P. G., Self G. J., Luttmann M. A., Hay D. W. Endothelin-1 receptor density, distribution, and function in human isolated asthmatic airways. Am J Respir Crit Care Med. 1995 Nov;152(5 Pt 1):1653–1658. doi: 10.1164/ajrccm.152.5.7582310. [DOI] [PubMed] [Google Scholar]
- Hay D. W., Luttmann M. A., Hubbard W. C., Undem B. J. Endothelin receptor subtypes in human and guinea-pig pulmonary tissues. Br J Pharmacol. 1993 Nov;110(3):1175–1183. doi: 10.1111/j.1476-5381.1993.tb13938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele R. G., Hayashi S., Hogg J. C., Paré P. D. Mechanisms of airway narrowing and hyperresponsiveness in viral respiratory tract infections. Am J Respir Crit Care Med. 1995 May;151(5):1659–1665. doi: 10.1164/ajrccm.151.5.7735630. [DOI] [PubMed] [Google Scholar]
- Hennet T., Ziltener H. J., Frei K., Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992 Aug 1;149(3):932–939. [PubMed] [Google Scholar]
- Henry P. J. Endothelin-1 (ET-1)-induced contraction in rat isolated trachea: involvement of ETA and ETB receptors and multiple signal transduction systems. Br J Pharmacol. 1993 Sep;110(1):435–441. doi: 10.1111/j.1476-5381.1993.tb13829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P. J., Goldie R. G. ETB but not ETA receptor-mediated contractions to endothelin-1 attenuated by respiratory tract viral infection in mouse airways. Br J Pharmacol. 1994 Aug;112(4):1188–1194. doi: 10.1111/j.1476-5381.1994.tb13209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P. J., Goldie R. G. Potentiation by endothelin-1 of cholinergic nerve-mediated contractions in mouse trachea via activation of ETB receptors. Br J Pharmacol. 1995 Feb;114(3):563–569. doi: 10.1111/j.1476-5381.1995.tb17176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P. J., Rigby P. J., Mackenzie J. S., Goldie R. G. Effect of respiratory tract viral infection on murine airway beta-adrenoceptor function, distribution and density. Br J Pharmacol. 1991 Dec;104(4):914–921. doi: 10.1111/j.1476-5381.1991.tb12526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui T., James A. F., Fujitani Y., Takimoto M., Okada T., Yamamura T., Urade Y. ETA and ETB receptors on single smooth muscle cells cooperate in mediating guinea pig tracheal contraction. Am J Physiol. 1994 Feb;266(2 Pt 1):L113–L124. doi: 10.1152/ajplung.1994.266.2.L113. [DOI] [PubMed] [Google Scholar]
- Jacoby D. B., Fryer A. D. Abnormalities in neural control of smooth muscle in virus-infected airways. Trends Pharmacol Sci. 1990 Oct;11(10):393–395. doi: 10.1016/0165-6147(90)90141-t. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Elkin R. B., Empey D. W., Jacobs L., Mills J., Nadel J. A. Bronchial hyperresponsiveness in normal subjects during attenuated influenza virus infection. Am Rev Respir Dis. 1991 Feb;143(2):358–361. doi: 10.1164/ajrccm/143.2.358. [DOI] [PubMed] [Google Scholar]
- Larsen G. L., Renz H., Loader J. E., Bradley K. L., Gelfand E. W. Airway response to electrical field stimulation in sensitized inbred mice. Passive transfer of increased responsiveness with peribronchial lymph nodes. J Clin Invest. 1992 Mar;89(3):747–752. doi: 10.1172/JCI115651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanske R. F., Jr, Dick E. C., Swenson C. A., Vrtis R. F., Busse W. W. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989 Jan;83(1):1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Hall W. J., Douglas R. G., Jr, Mudholkar G. S., Speers D. M., Patel K. Airway hyperreactivity and peripheral airway dysfunction in influenza A infection. Am Rev Respir Dis. 1978 Aug;118(2):295–303. doi: 10.1164/arrd.1978.118.2.295. [DOI] [PubMed] [Google Scholar]
- MacCumber M. W., Ross C. A., Glaser B. M., Snyder S. H. Endothelin: visualization of mRNAs by in situ hybridization provides evidence for local action. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7285–7289. doi: 10.1073/pnas.86.18.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay K. O., Armour C. L., Black J. L. Endothelin-3 increases transmission in the rabbit pulmonary parasympathetic nervous system. J Cardiovasc Pharmacol. 1993;22 (Suppl 8):S181–S184. doi: 10.1097/00005344-199322008-00049. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Uchida Y., Endo T., Ninomiya H., Nomura A., Sakamoto T., Goto Y., Haraoka S., Shimokama T., Watanabe T. The induction of cell differentiation and polarity of tracheal epithelium cultured on the amniotic membrane. Biochem Biophys Res Commun. 1995 May 16;210(2):302–309. doi: 10.1006/bbrc.1995.1661. [DOI] [PubMed] [Google Scholar]
- Rozengurt N., Springall D. R., Polak J. M. Localization of endothelin-like immunoreactivity in airway epithelium of rats and mice. J Pathol. 1990 Jan;160(1):5–8. doi: 10.1002/path.1711600104. [DOI] [PubMed] [Google Scholar]
- Springall D. R., Howarth P. H., Counihan H., Djukanovic R., Holgate S. T., Polak J. M. Endothelin immunoreactivity of airway epithelium in asthmatic patients. Lancet. 1991 Mar 23;337(8743):697–701. doi: 10.1016/0140-6736(91)90279-x. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout A. J., Hofman G., Woutersen-Van Nijnanten F. M., Nijkamp F. P. 5-HT1-like receptors mediate potentiation of cholinergic nerve-mediated contraction of isolated mouse trachea. Eur J Pharmacol. 1991 Dec 17;209(3):237–244. doi: 10.1016/0014-2999(91)90175-p. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Williams K., Mackenzie J. S. Influenza infections during pregnancy in the mouse. J Hyg (Lond) 1977 Oct;79(2):249–257. doi: 10.1017/s0022172400053067. [DOI] [PMC free article] [PubMed] [Google Scholar]


