Abstract
1. Binding of D,L-(E)-2-amino-4-[3H]-propyl-5-phosphono-3-pentenoic acid ([3H]-CGP 39653), a high affinity, selective antagonist at the glutamate site of the N-methyl-D-aspartate (NMDA) receptor, was investigated in rat brain by means of receptor binding and quantitative autoradiography techniques. 2. [3H]-CGP 39653 interacted with striatal and cerebellar membranes in a saturable manner and to a single binding site, with KD values of 15.5 nM and 10.0 nM and receptor binding densities (Bmax values) of 3.1 and 0.5 pmol mg-1 protein, respectively. These KD values were not significantly different from that previously reported in the cerebral cortex (10.7 nM). 3. Displacement analyses of [3H]-CGP 39653 in striatum and cerebellum, performed with L-glutamic acid, 3-((+/-)-2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) and glycine showed a pharmacological profile similar to that reported in the cerebral cortex. L-Glutamic acid and CPP produced complete displacement of specific binding with Ki values not significantly different from the cerebral cortex. Glycine inhibited [3H]CGP 39653 binding with shallow, biphasic curves, characterized by a high and a low affinity component. Furthermore, glycine discriminated between these regions (P < 0.005, one-way ANOVA), since the apparent Ki of the high affinity component of the glycine inhibition curve (KiH) was significantly lower (Fisher's protected LSD) in the striatum than the cortex (33 nM and 104 nM, respectively). 4. Regional binding of [3H]-CGP 39653 to horizontal sections of rat brain revealed a heterogeneous distribution of binding sites, similar to that reported for other radiolabelled antagonists at the NMDA site (D-2-[3H]-amino-5-phosphonopentanoic acid ([3H]-D-AP5) and [3H]-CPP). High values of binding were detected in the hippocampal formation, cerebral cortex and thalamus, with low levels in striatum and cerebellum. 5. [3H]-CGP 39653 binding was inhibited by increasing concentrations of L-glutamic acid, CPP and glycine. L-Glutamic acid and CPP completely displaced specific binding in all regions tested, with similar IC50 values throughout. Similarly, glycine was able to inhibit the binding in all areas considered: 10 microM and 1 mM glycine reduced the binding to 80% and 65% of control (average between areas) respectively. The percentage of specific [3H]-CGP 39653 binding inhibited by 1 mM glycine varied among regions (P < 0.05, two-ways ANOVA). Multiple comparison, performed by Fisher's protected LSD method, showed that the inhibition was lower in striatum (72% of control), with respect to cortex (66% of control) and hippocampal formation (58% of control). 6. The inhibitory action of 10 microM glycine was reversed by 100 microM 7-chloro-kynurenic acid (7-CKA), a competitive antagonist of the glycine site of the NMDA receptor channel complex, in all areas tested. Moreover, reversal by 7-CKA was not the same in all regions (P < 0.05, two-ways ANOVA). In fact, in the presence of 10 microM glycine and 100 microM 7-KCA, specific [3H]-CGP 39653 binding in the striatum was 131% of control, which was significantly greater (Fisher's protected LSD) than binding in the hippocampus and the thalamus (104% and 112% of control, respectively). 7. These results demonstrate that [3H]-CGP 39653 binding can be inhibited by glycine in rat brain regions containing NMDA receptors; moreover, they suggest the existence of regionally distinct NMDA receptor subtypes with a different allosteric mechanism of [3H]-CGP 39653 binding modulation through the associated glycine site.
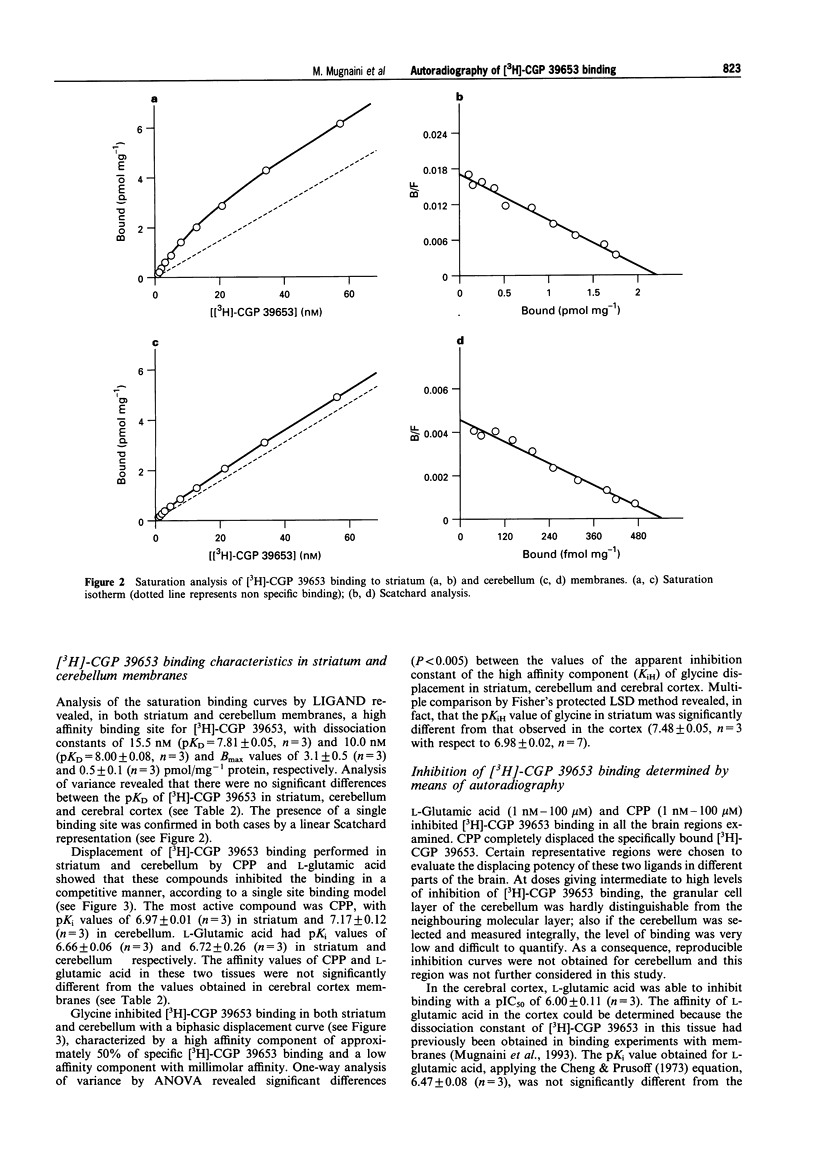
Full text
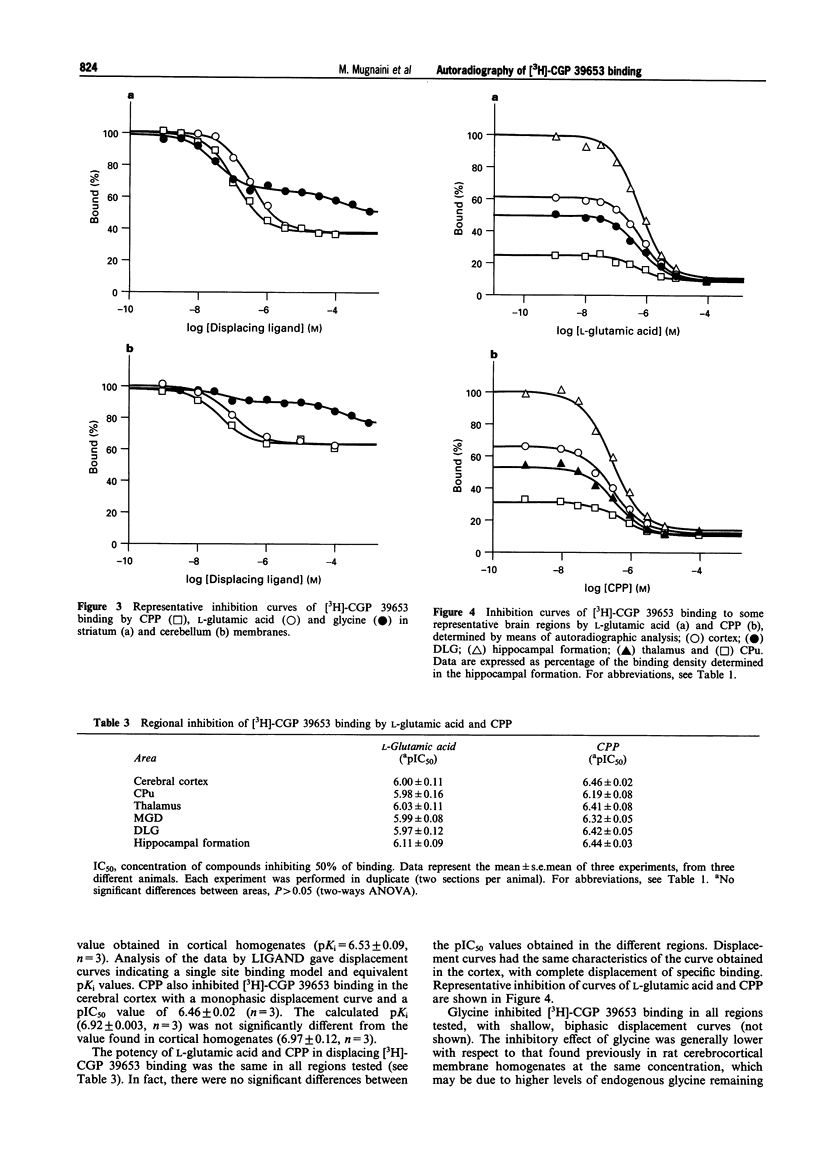
PDF

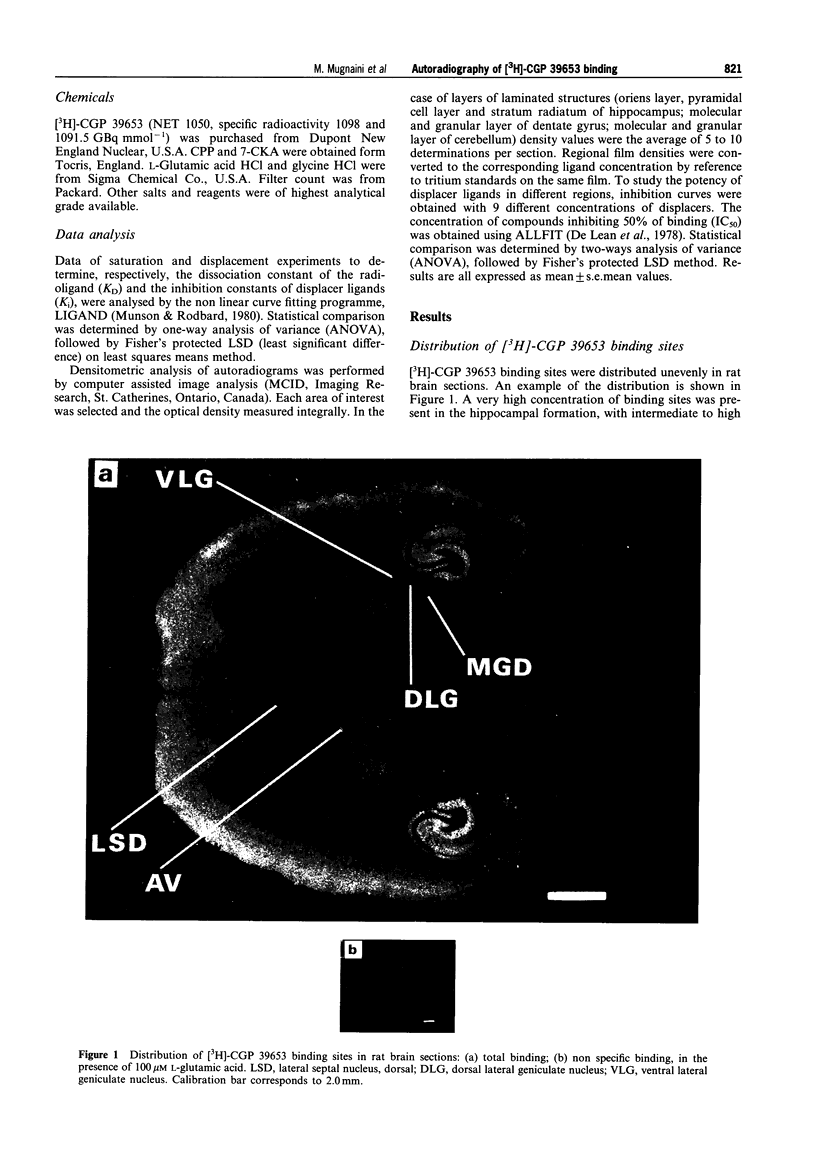
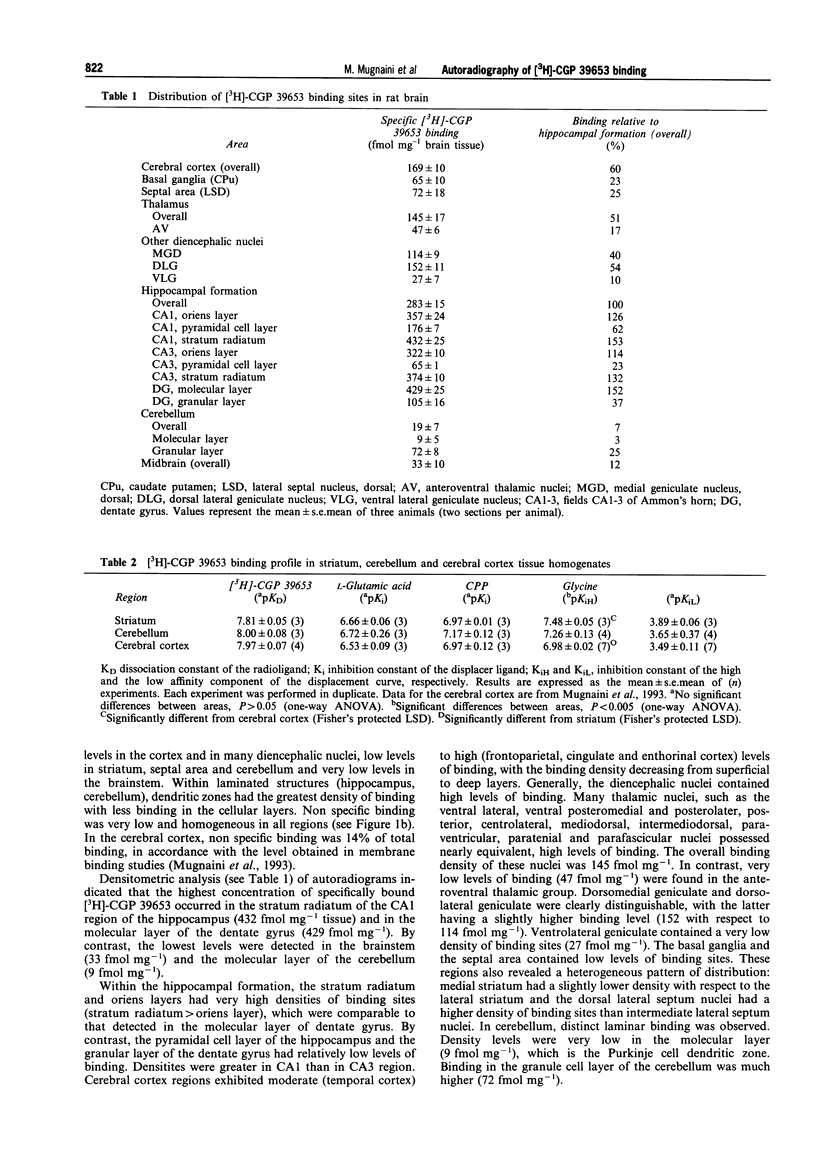



Images in this article
Selected References
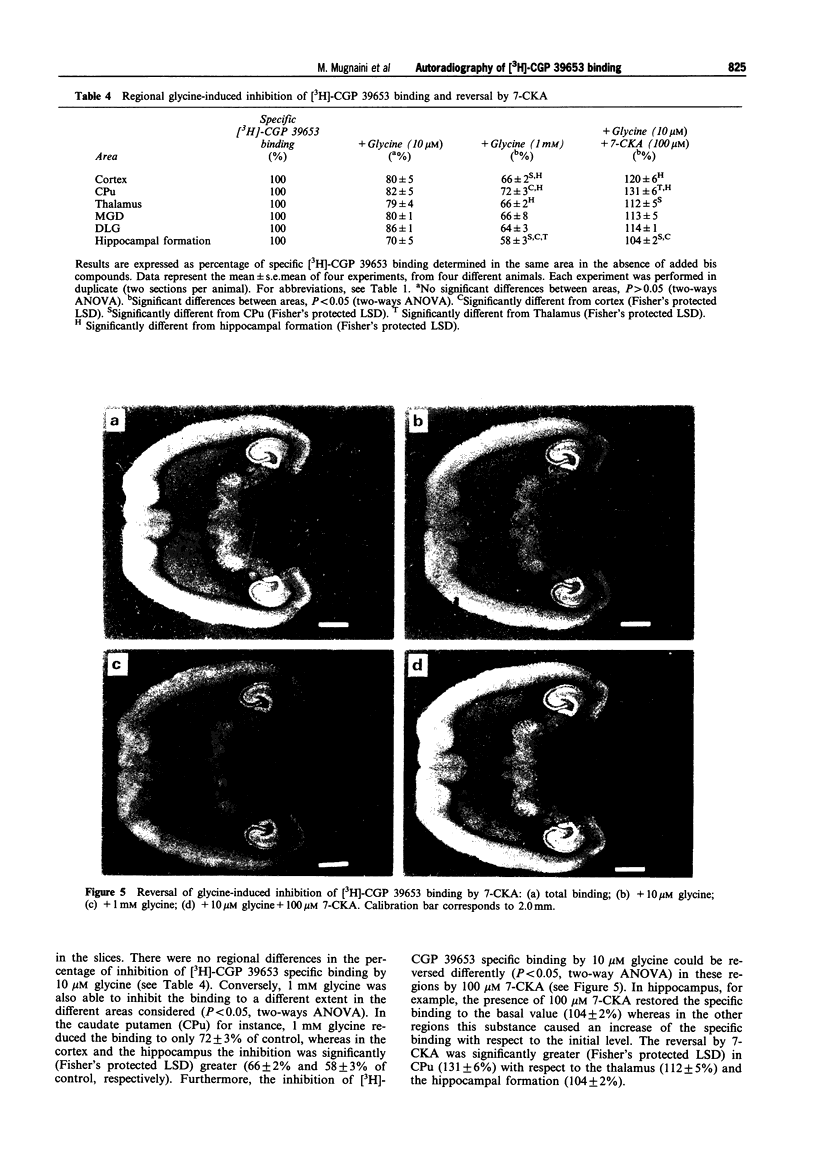
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki C., Venkatesan C., Go C. G., Mong J. A., Dawson T. M. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci. 1994 Sep;14(9):5202–5222. doi: 10.1523/JNEUROSCI.14-09-05202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton J. A., Stemsrud K., Monaghan D. T. Identification of a novel N-methyl-D-aspartate receptor population in the rat medial thalamus. J Neurochem. 1992 Aug;59(2):754–757. doi: 10.1111/j.1471-4159.1992.tb09433.x. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Wong E. H., Hudson A. L. Quantitative autoradiography of [3H]-MK-801 binding sites in mammalian brain. Br J Pharmacol. 1988 Apr;93(4):944–954. doi: 10.1111/j.1476-5381.1988.tb11484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller A. L., Larson H. C., Schneider B. E., Beaton J. A., Morrisett R. A., Monaghan D. T. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994 Sep;14(9):5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazot P. L., Coleman S. K., Cik M., Stephenson F. A. Molecular characterization of N-methyl-D-aspartate receptors expressed in mammalian cells yields evidence for the coexistence of three subunit types within a discrete receptor molecule. J Biol Chem. 1994 Sep 30;269(39):24403–24409. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990 Jul;11(7):290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Ebert B., Wong E. H., Krogsgaard-Larsen P. Identification of a novel NMDA receptor in rat cerebellum. Eur J Pharmacol. 1991 Sep 12;208(1):49–52. doi: 10.1016/0922-4106(91)90050-r. [DOI] [PubMed] [Google Scholar]
- Fadda E., Danysz W., Wroblewski J. T., Costa E. Glycine and D-serine increase the affinity of N-methyl-D-aspartate sensitive glutamate binding sites in rat brain synaptic membranes. Neuropharmacology. 1988 Nov;27(11):1183–1185. doi: 10.1016/0028-3908(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Olpe H. R., Pozza M. F., Baud J., Steinmann M., Schmutz M., Portet C., Baumann P., Thedinga K., Bittiger H. CGP 37849 and CGP 39551: novel and potent competitive N-methyl-D-aspartate receptor antagonists with oral activity. Br J Pharmacol. 1990 Apr;99(4):791–797. doi: 10.1111/j.1476-5381.1990.tb13008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood S., Foster A. C., Kemp J. A. The pharmacological specificity of N-methyl-D-aspartate receptors in rat cerebral cortex: correspondence between radioligand binding and electrophysiological measurements. Br J Pharmacol. 1991 Jun;103(2):1385–1392. doi: 10.1111/j.1476-5381.1991.tb09799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., Boulter J., Maron C., Beasley L., Sullivan J., Pecht G., Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993 May;10(5):943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- Honoré T., Drejer J., Nielsen E. O., Watkins J. C., Olverman H. J., Nielsen M. Molecular target size analyses of the NMDA-receptor complex in rat cortex. Eur J Pharmacol. 1989 Aug 15;172(3):239–247. doi: 10.1016/0922-4106(89)90054-0. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Nagasawa M., Mori H., Araki K., Sakimura K., Watanabe M., Inoue Y., Mishina M. Cloning and expression of the epsilon 4 subunit of the NMDA receptor channel. FEBS Lett. 1992 Nov 16;313(1):34–38. doi: 10.1016/0014-5793(92)81178-o. [DOI] [PubMed] [Google Scholar]
- Ishii T., Moriyoshi K., Sugihara H., Sakurada K., Kadotani H., Yokoi M., Akazawa C., Shigemoto R., Mizuno N., Masu M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993 Feb 5;268(4):2836–2843. [PubMed] [Google Scholar]
- Jarvis M. F., Murphy D. E., Williams M. Quantitative autoradiographic localization of NMDA receptors in rat brain using [3H]CPP: comparison with [3H]TCP binding sites. Eur J Pharmacol. 1987 Sep 2;141(1):149–152. doi: 10.1016/0014-2999(87)90423-7. [DOI] [PubMed] [Google Scholar]
- Komuro H., Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993 Apr 2;260(5104):95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Laurie D. J., Seeburg P. H. Ligand affinities at recombinant N-methyl-D-aspartate receptors depend on subunit composition. Eur J Pharmacol. 1994 Aug 16;268(3):335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Lynch D. R., Anegawa N. J., Verdoorn T., Pritchett D. B. N-methyl-D-aspartate receptors: different subunit requirements for binding of glutamate antagonists, glycine antagonists, and channel-blocking agents. Mol Pharmacol. 1994 Mar;45(3):540–545. [PubMed] [Google Scholar]
- Maragos W. F., Penney J. B., Young A. B. Anatomic correlation of NMDA and 3H-TCP-labeled receptors in rat brain. J Neurosci. 1988 Feb;8(2):493–501. doi: 10.1523/JNEUROSCI.08-02-00493.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. W., Penney J. B., Johnston M. V., Young A. B. Characterization and regional distribution of strychnine-insensitive [3H]glycine binding sites in rat brain by quantitative receptor autoradiography. Neuroscience. 1990;35(3):653–668. doi: 10.1016/0306-4522(90)90336-3. [DOI] [PubMed] [Google Scholar]
- Meguro H., Mori H., Araki K., Kushiya E., Kutsuwada T., Yamazaki M., Kumanishi T., Arakawa M., Sakimura K., Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992 May 7;357(6373):70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Beaton J. A. Quinolinate differentiates between forebrain and cerebellar NMDA receptors. Eur J Pharmacol. 1991 Feb 26;194(1):123–125. doi: 10.1016/0014-2999(91)90134-c. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Olverman H. J., Nguyen L., Watkins J. C., Cotman C. W. Two classes of N-methyl-D-aspartate recognition sites: differential distribution and differential regulation by glycine. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9836–9840. doi: 10.1073/pnas.85.24.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Yao D., Olverman H. J., Watkins J. C., Cotman C. W. Autoradiography of D-2-[3H]amino-5-phosphonopentanoate binding sites in rat brain. Neurosci Lett. 1984 Dec 21;52(3):253–258. doi: 10.1016/0304-3940(84)90170-8. [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Mugnaini M., Giberti A., Ratti E., van Amsterdam F. T. Allosteric modulation of [3H]CGP 39653 binding by glycine in rat brain. J Neurochem. 1993 Oct;61(4):1492–1497. doi: 10.1111/j.1471-4159.1993.tb13644.x. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Murphy D. E., Hutchison A. J., Hurt S. D., Williams M., Sills M. A. Characterization of the binding of [3H]-CGS 19755: a novel N-methyl-D-aspartate antagonist with nanomolar affinity in rat brain. Br J Pharmacol. 1988 Nov;95(3):932–938. doi: 10.1111/j.1476-5381.1988.tb11723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Wang Y. X., Wenthold R. J. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci. 1994 Oct;14(10):6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia R. S., Yokotani N., Wenthold R. J. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994 Feb;14(2):667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. H., Cowburn R. F., Alasuzoff I., Briggs R. S., Roberts P. J. Heterogeneity of NMDA receptors labelled with [3H]3-((+-)-2-carboxypiperazin-4-yl) propyl-1-phosphonic acid ([3H]CPP): receptor status in Alzheimer's disease brains. Eur J Pharmacol. 1992 Mar 12;225(3):195–201. doi: 10.1016/0922-4106(92)90020-v. [DOI] [PubMed] [Google Scholar]
- Priestley T., Laughton P., Myers J., Le Bourdellés B., Kerby J., Whiting P. J. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995 Nov;48(5):841–848. [PubMed] [Google Scholar]
- Reynolds I. J., Palmer A. M. Regional variations in [3H]MK801 binding to rat brain N-methyl-D-aspartate receptors. J Neurochem. 1991 May;56(5):1731–1740. doi: 10.1111/j.1471-4159.1991.tb02074.x. [DOI] [PubMed] [Google Scholar]
- Sakurai S. Y., Penney J. B., Young A. B. Regionally distinct N-methyl-D-aspartate receptors distinguished by quantitative autoradiography of [3H]MK-801 binding in rat brain. J Neurochem. 1993 Apr;60(4):1344–1353. doi: 10.1111/j.1471-4159.1993.tb03295.x. [DOI] [PubMed] [Google Scholar]
- Sills M. A., Fagg G., Pozza M., Angst C., Brundish D. E., Hurt S. D., Wilusz E. J., Williams M. [3H]CGP 39653: a new N-methyl-D-aspartate antagonist radioligand with low nanomolar affinity in rat brain. Eur J Pharmacol. 1991 Jan 3;192(1):19–24. doi: 10.1016/0014-2999(91)90063-v. [DOI] [PubMed] [Google Scholar]
- Sugihara H., Moriyoshi K., Ishii T., Masu M., Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun. 1992 Jun 30;185(3):826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Bain C. J., Le Bourdelles B., Whiting P. J., Kemp J. A. Preferential co-assembly of recombinant NMDA receptors composed of three different subunits. Neuroreport. 1993 Sep 30;4(12):1347–1349. doi: 10.1097/00001756-199309150-00015. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Inoue Y., Sakimura K., Mishina M. Distinct distributions of five N-methyl-D-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol. 1993 Dec 15;338(3):377–390. doi: 10.1002/cne.903380305. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993 Oct;44(4):851–859. [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A. Sites for antagonism on the N-methyl-D-aspartate receptor channel complex. Annu Rev Pharmacol Toxicol. 1991;31:401–425. doi: 10.1146/annurev.pa.31.040191.002153. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Mori H., Araki K., Mori K. J., Mishina M. Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett. 1992 Mar 23;300(1):39–45. doi: 10.1016/0014-5793(92)80160-i. [DOI] [PubMed] [Google Scholar]
- Yoneda Y., Ogita K. Heterogeneity of the N-methyl-D-aspartate receptor ionophore complex in rat brain, as revealed by ligand binding techniques. J Pharmacol Exp Ther. 1991 Oct;259(1):86–96. [PubMed] [Google Scholar]
- Zuo P., Ogita K., Han D., Yoneda Y. Comparative studies on binding of 3 different ligands to the N-methyl-D-aspartate recognition domain in brain synaptic membranes treated with Triton X-100. Brain Res. 1993 Apr 23;609(1-2):253–261. doi: 10.1016/0006-8993(93)90880-v. [DOI] [PubMed] [Google Scholar]
- van Amsterdam F. T., Giberti A., Mugnaini M., Ratti E. 3-[(+-)-2-carboxypiperazin-4-yl]propyl-1-phosphonic acid recognizes two N-methyl-D-aspartate binding sites in rat cerebral cortex membranes. J Neurochem. 1992 Nov;59(5):1850–1855. doi: 10.1111/j.1471-4159.1992.tb11019.x. [DOI] [PubMed] [Google Scholar]