Abstract
1. The effects of addition of Cu2+ and chelation of Cu2+ were studied on relaxations in response to S-nitrosothiols and on relaxations to non-adrenergic non-cholinergic (NANC) nerve stimulation, nitric oxide (NO) and glyceryl trinitrate (GTN) in the rat gastric fundus. 2. The S-nitrosothiols S-nitroso-L-cysteine (NOCys, 1-300 nM), S-nitrosoglutathione (GSNO, 0.01-3 microM) and S-nitroso-N-acetyl-D,L-penicillamine (SNAP, 0.01-3 microM) induced concentration-dependent relaxations of the rat gastric fundus muscle strip. The relaxant potencies of the S-nitrosothiols were NOCys > SNAP > GSNO. Relaxations to NOCys were transient and comparable to those to NANC nerve stimulation and NO whereas relaxations to GSNO and SNAP were sustained. The relaxations to NOCys, GSNO and SNAP were significantly and concentration-dependently enhanced by CuSO4 (3-30 microM). The order of relaxant potency in the presence of CuSO4 was reversed to GSNO approximately SNAP > NOCys. 3. In the presence but not in the absence of 0.1 microM GSNO, CuSO4 (1 microM) induced a rapid and transient relaxation which was inhibited by the superoxide radical generator, pyrogallol (30 microM). CuCl2 but not FeSO4 mimicked the effect of CuSO4. 4. Electrical stimulation (0.5-8 Hz) of the rat gastric fundus strips induced frequency-dependent relaxations which were previously shown to be nitrergic in nature and which were not affected by CuSO4 (3-30 microM). Relaxations to NO (3-100 nM) and GTN (0.01-1 microM) were not affected by 3 and 10 microM CuSO4 but were inhibited by 30 microM CuSO4. 5. The Cu2+ chelator, bathocuproine (3-30 microM) significantly and concentration-dependently inhibited the relaxations to NOCys (0.01-3 microM), GSNO (0.01-10 microM) and SNAP (0.01-3 microM). The inhibitory effect of 10 microM bathocuproine was reversed by 3 microM CuSO4. 6. Bathocuproine (3-30 microM) had no effect on the relaxations to NANC nerve stimulation (0.5-8 Hz) or on the concentration-response curve to NO (0.01-0.3 microM), whereas relaxations to GTN (0.01-1 microM) were significantly inhibited by 30 microM bathocuproine. 7. From these results we conclude that relaxations to S-nitrosothiols and to nitrergic stimulation of the rat gastric fundus are differentially affected by addition and chelation of Cu2+, suggesting that the nitrergic NANC neurotransmitter in the rat gastric fundus is not an S-nitrosothiol but is more likely to be free nitric oxide.
Full text
PDF
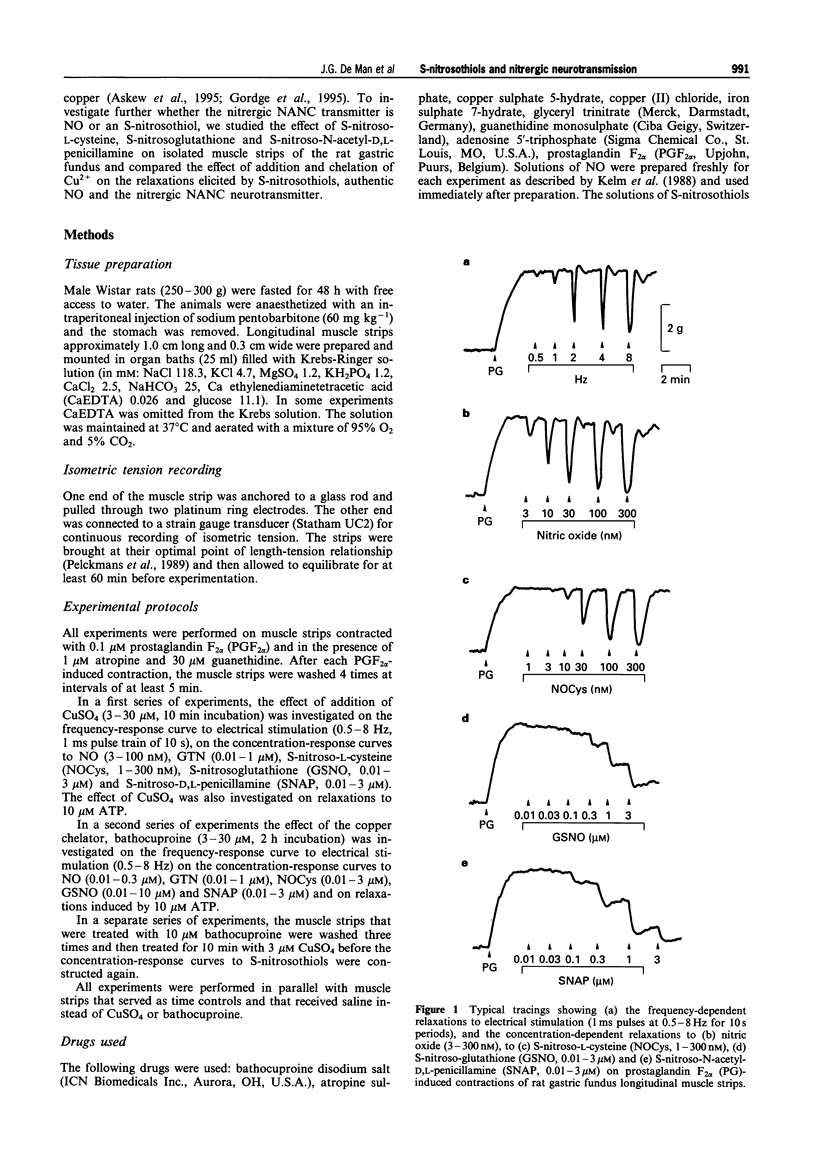
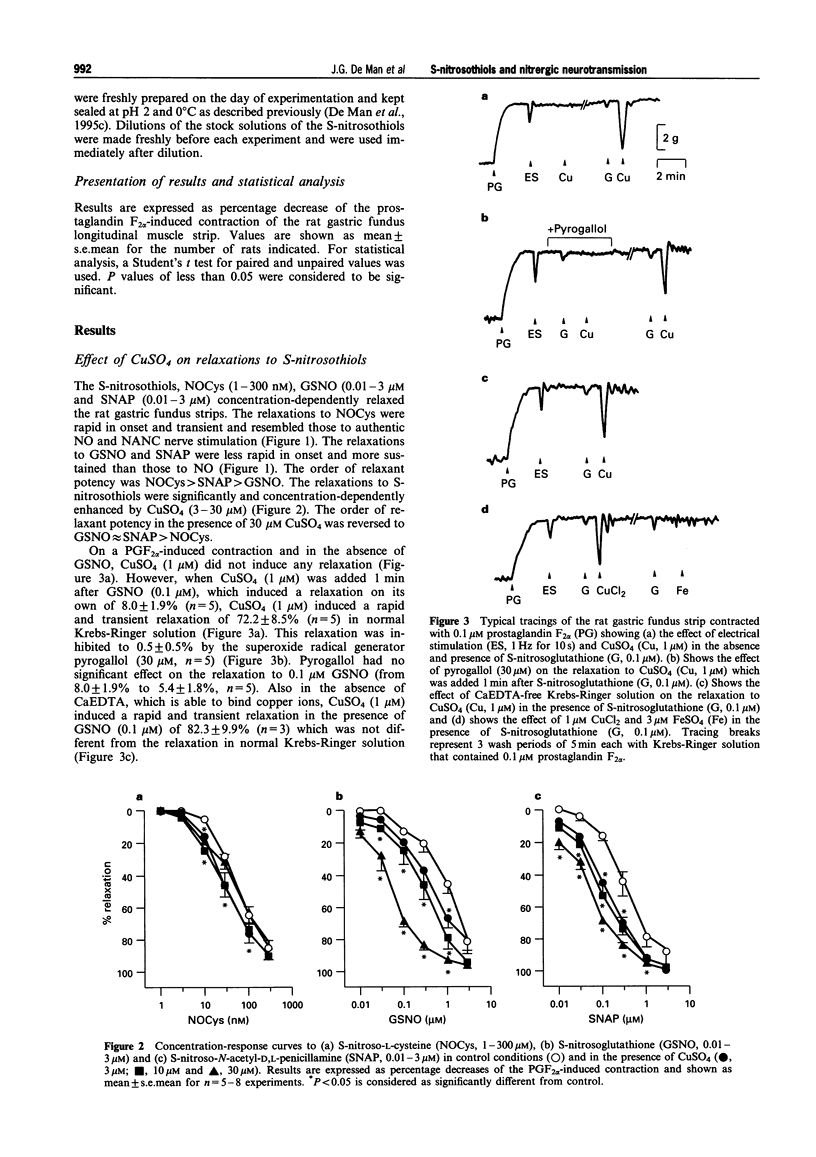
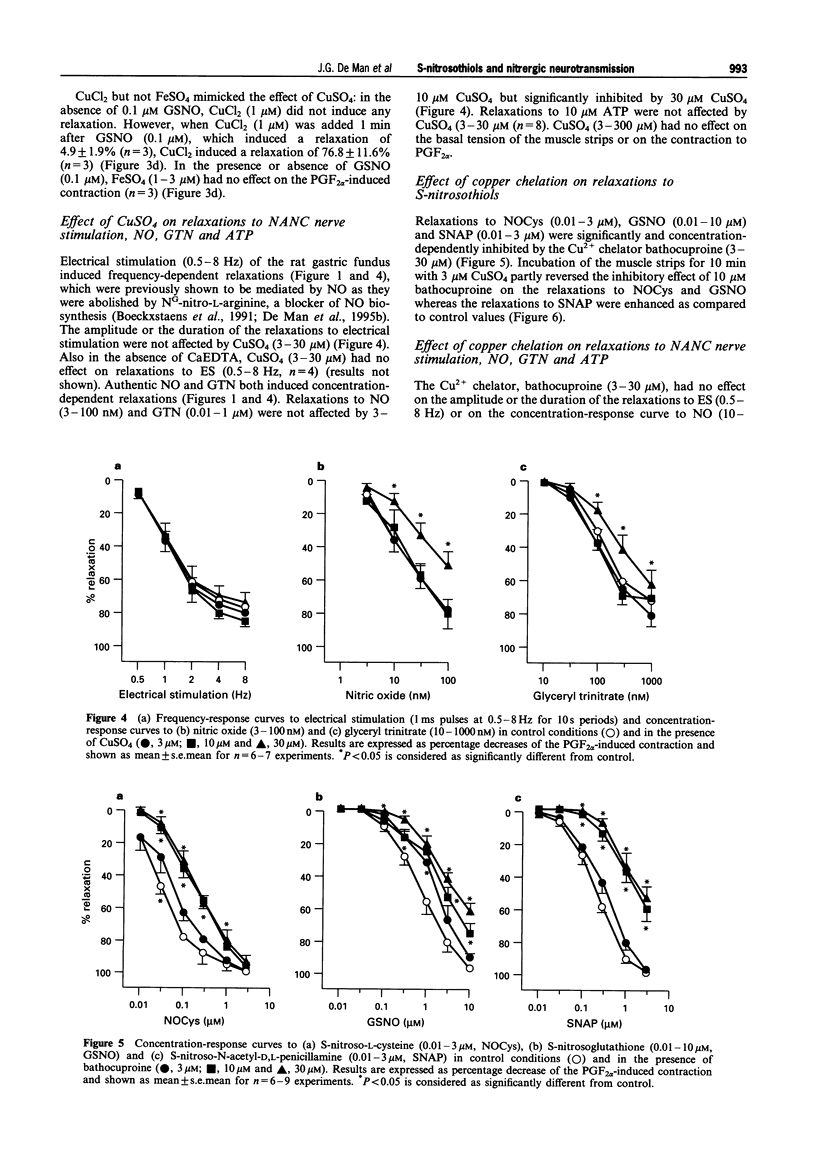
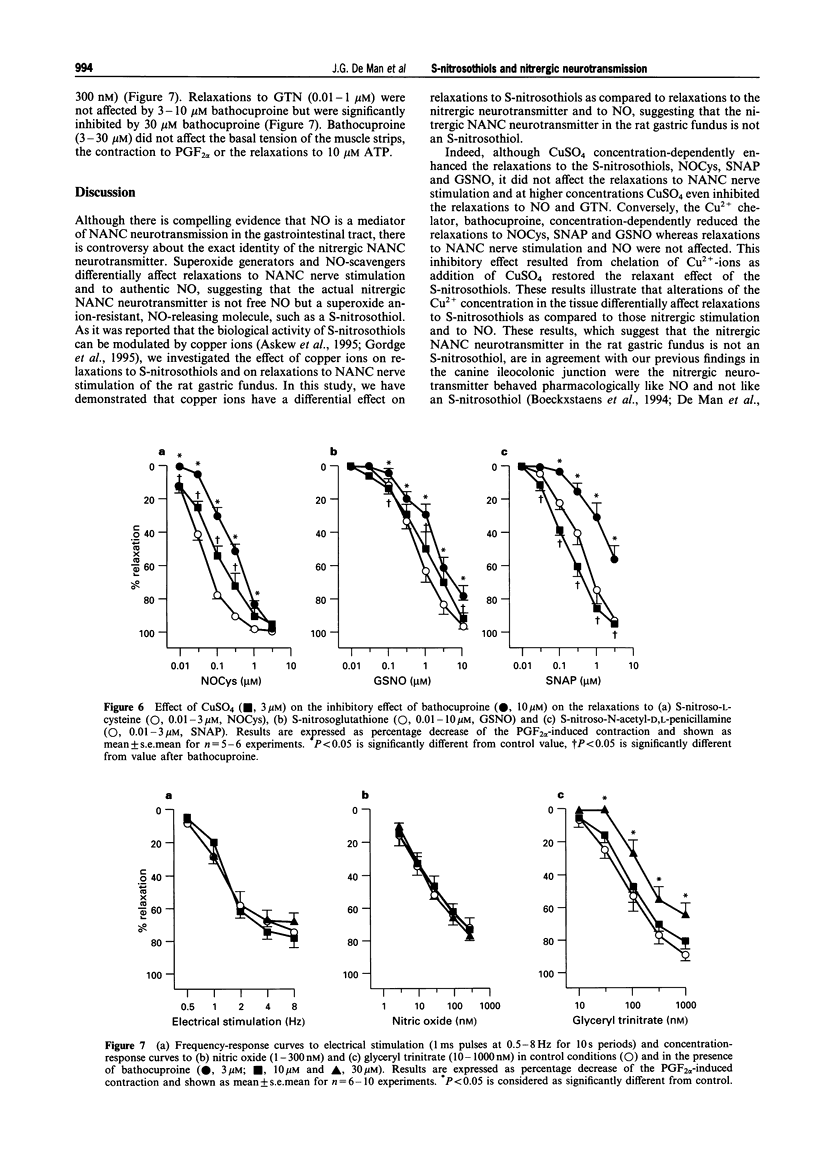


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbier A. J., Lefebvre R. A. Effect of 3-isobutyl-1-methylxanthine and zaprinast on non-adrenergic non-cholinergic relaxation in the rat gastric fundus. Eur J Pharmacol. 1992 Jan 21;210(3):315–323. doi: 10.1016/0014-2999(92)90421-y. [DOI] [PubMed] [Google Scholar]
- Barbier A. J., Lefebvre R. A. Effect of LY 83583 on relaxation induced by non-adrenergic non-cholinergic nerve stimulation and exogenous nitric oxide in the rat gastric fundus. Eur J Pharmacol. 1992 Aug 25;219(2):331–334. doi: 10.1016/0014-2999(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Barbier A. J., Lefebvre R. A. Influence of S-nitrosothiols and nitrate tolerance in the rat gastric fundus. Br J Pharmacol. 1994 Apr;111(4):1280–1286. doi: 10.1111/j.1476-5381.1994.tb14884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckxstaens G. E., De Man J. G., De Winter B. Y., Herman A. G., Pelckmans P. A. Pharmacological similarity between nitric oxide and the nitrergic neurotransmitter in the canine ileocolonic junction. Eur J Pharmacol. 1994 Oct 13;264(1):85–89. doi: 10.1016/0014-2999(94)90640-8. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., Bogers J. J., Bult H., De Man J. G., Oosterbosch L., Herman A. G., Van Maercke Y. M. Release of nitric oxide upon stimulation of nonadrenergic noncholinergic nerves in the rat gastric fundus. J Pharmacol Exp Ther. 1991 Feb;256(2):441–447. [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., Bult H., De Man J. G., Herman A. G., Van Maercke Y. M. Non-adrenergic non-cholinergic relaxation mediated by nitric oxide in the canine ileocolonic junction. Eur J Pharmacol. 1990 Nov 6;190(1-2):239–246. doi: 10.1016/0014-2999(90)94132-h. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- De Man J. G., Boeckxstaens G. E., De Winter B. Y., Moreels T. G., Herman A. G., Pelckmans P. A. Inhibition of non-adrenergic non-cholinergic relaxations by nitric oxide donors. Eur J Pharmacol. 1995 Oct 24;285(3):269–274. doi: 10.1016/0014-2999(95)00420-p. [DOI] [PubMed] [Google Scholar]
- De Man J. G., Boeckxstaens G. E., De Winter B. Y., Moreels T. G., Misset M. E., Herman A. G., Pelckmans P. A. Comparison of the pharmacological profile of S-nitrosothiols, nitric oxide and the nitrergic neurotransmitter in the canine ileocolonic junction. Br J Pharmacol. 1995 Mar;114(6):1179–1184. doi: 10.1111/j.1476-5381.1995.tb13331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio P., Cheeseman K. H., Slater T. F. The reactivity of the SH group of bovine serum albumin with free radicals. Free Radic Res Commun. 1991;14(4):253–262. doi: 10.3109/10715769109088954. [DOI] [PubMed] [Google Scholar]
- Feelisch M., te Poel M., Zamora R., Deussen A., Moncada S. Understanding the controversy over the identity of EDRF. Nature. 1994 Mar 3;368(6466):62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- Gibson A., Babbedge R., Brave S. R., Hart S. L., Hobbs A. J., Tucker J. F., Wallace P., Moore P. K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br J Pharmacol. 1992 Nov;107(3):715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. The effects of pyrogallol and hydroquinone on the response to NANC nerve stimulation in the rat anococcygeus and the bovine retractor penis muscles. Br J Pharmacol. 1990 Jan;99(1):194–196. doi: 10.1111/j.1476-5381.1990.tb14677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordge M. P., Meyer D. J., Hothersall J., Neild G. H., Payne N. N., Noronha-Dutra A. Copper chelation-induced reduction of the biological activity of S-nitrosothiols. Br J Pharmacol. 1995 Mar;114(5):1083–1089. doi: 10.1111/j.1476-5381.1995.tb13317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. J., Tucker J. F., Gibson A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br J Pharmacol. 1991 Nov;104(3):645–650. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Iversen H. H., Gustafsson L. E., Leone A. M., Wiklund N. P. Smooth muscle relaxing effects of NO, nitrosothiols and a nerve-induced relaxing factor released in guinea-pig colon. Br J Pharmacol. 1994 Dec;113(4):1088–1092. doi: 10.1111/j.1476-5381.1994.tb17107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson K. M., Reid J. J., Rand M. J. Hydroxocobalamin and haemoglobin differentiate between exogenous and neuronal nitric oxide in the rat gastric fundus. Eur J Pharmacol. 1995 Mar 6;275(2):145–152. doi: 10.1016/0014-2999(94)00762-v. [DOI] [PubMed] [Google Scholar]
- Kamitani T. Inhibitory effect of Cu2+ on nitroglycerin-induced vasodilation. Eur J Pharmacol. 1984 Apr 20;100(2):145–154. doi: 10.1016/0014-2999(84)90216-4. [DOI] [PubMed] [Google Scholar]
- Kelm M., Feelisch M., Spahr R., Piper H. M., Noack E., Schrader J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):236–244. doi: 10.1016/0006-291x(88)90675-4. [DOI] [PubMed] [Google Scholar]
- Kerr S. W., Buchanan L. V., Bunting S., Mathews W. R. Evidence that S-nitrosothiols are responsible for the smooth muscle relaxing activity of the bovine retractor penis inhibitory factor. J Pharmacol Exp Ther. 1992 Oct;263(1):285–292. [PubMed] [Google Scholar]
- Kitamura K., Lian Q., Carl A., Kuriyama H. S-nitrosocysteine, but not sodium nitroprusside, produces apamin-sensitive hyperpolarization in rat gastric fundus. Br J Pharmacol. 1993 Jun;109(2):415–423. doi: 10.1111/j.1476-5381.1993.tb13585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley E., Gibson A. Inhibition of relaxations to nitrergic stimulation of the mouse anococcygeus by duroquinone. Br J Pharmacol. 1995 Dec;116(8):3231–3236. doi: 10.1111/j.1476-5381.1995.tb15129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Gillespie J. S., Martin W. Non-adrenergic, non-cholinergic relaxation of the bovine retractor penis muscle: role of S-nitrosothiols. Br J Pharmacol. 1994 Apr;111(4):1287–1295. doi: 10.1111/j.1476-5381.1994.tb14885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., McAllister K. H., Paisley K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacology. 1994 Nov;33(11):1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Mathews W. R., Kerr S. W. Biological activity of S-nitrosothiols: the role of nitric oxide. J Pharmacol Exp Ther. 1993 Dec;267(3):1529–1537. [PubMed] [Google Scholar]
- Needleman P., Jakschik B., Johnson E. M., Jr Sulfhydryl requirement for relaxation of vascular smooth muscle. J Pharmacol Exp Ther. 1973 Nov;187(2):324–331. [PubMed] [Google Scholar]
- Pelckmans P. A., Boeckxstaens G. E., Van Maercke Y. M., Herman A. G., Verbeuren T. J. Acetylcholine is an indirect inhibitory transmitter in the canine ileocolonic junction. Eur J Pharmacol. 1989 Nov 7;170(3):235–242. doi: 10.1016/0014-2999(89)90544-x. [DOI] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDRF. Br J Pharmacol. 1995 Sep;116(2):1906–1910. doi: 10.1111/j.1476-5381.1995.tb16681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Ward S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992 Mar;262(3 Pt 1):G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Stark M. E., Szurszewski J. H. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992 Dec;103(6):1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- Thornbury K. D., Ward S. M., Dalziel H. H., Carl A., Westfall D. P., Sanders K. M. Nitric oxide and nitrosocysteine mimic nonadrenergic, noncholinergic hyperpolarization in canine proximal colon. Am J Physiol. 1991 Sep;261(3 Pt 1):G553–G557. doi: 10.1152/ajpgi.1991.261.3.G553. [DOI] [PubMed] [Google Scholar]
- Wiklund N. P., Leone A. M., Gustafsson L. E., Moncada S. Release of nitric oxide evoked by nerve stimulation in guinea-pig intestine. Neuroscience. 1993 Apr;53(3):607–611. doi: 10.1016/0306-4522(93)90609-j. [DOI] [PubMed] [Google Scholar]


