Abstract
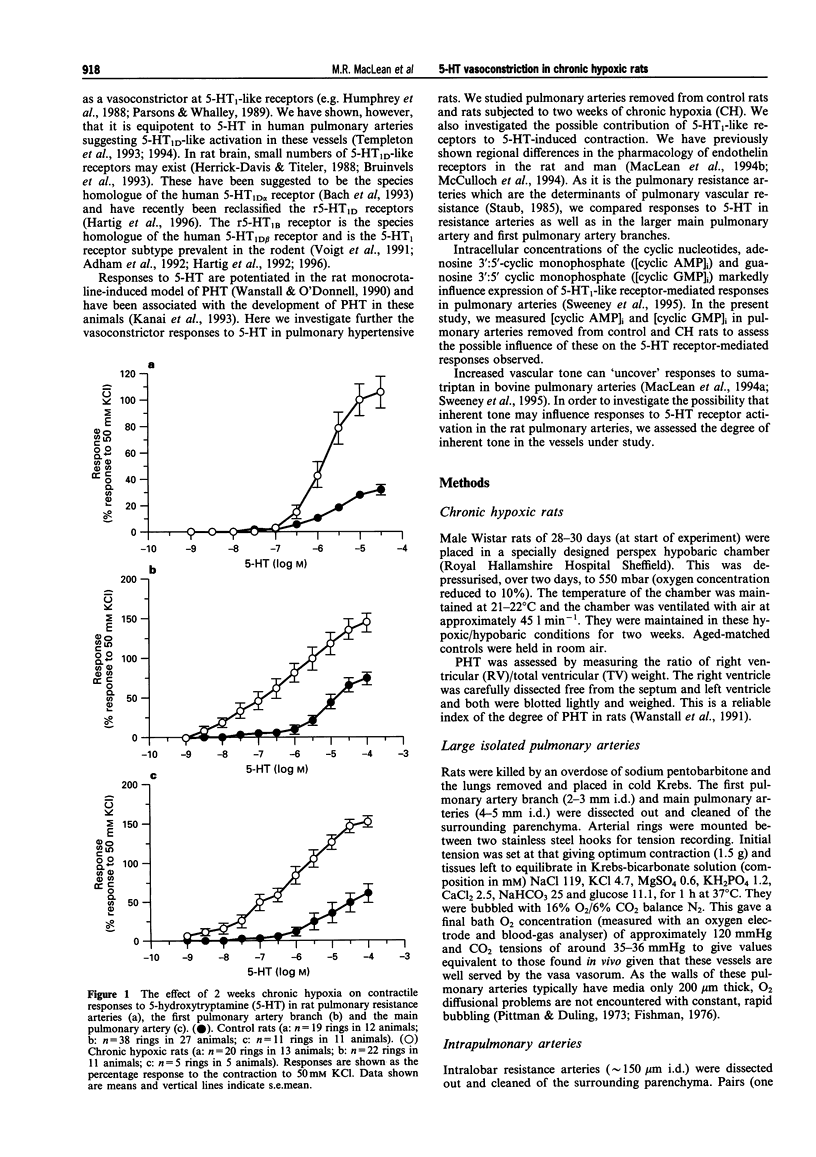
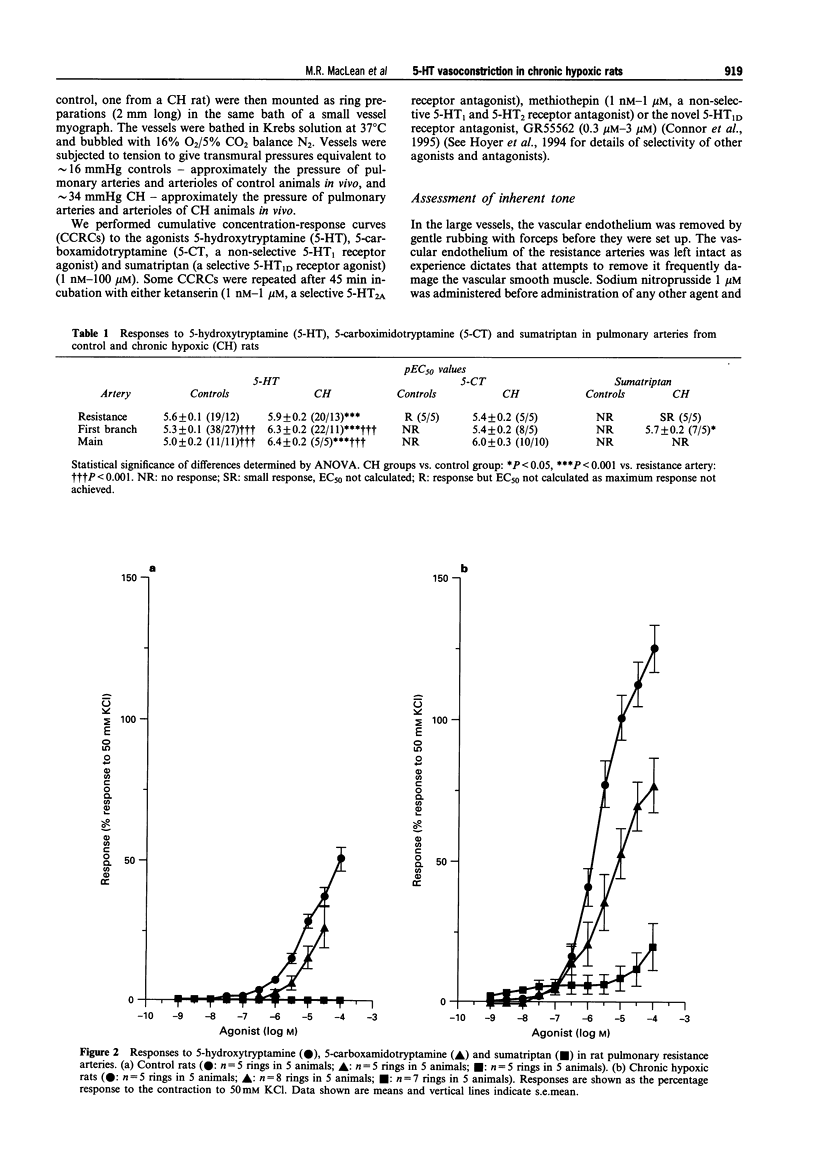
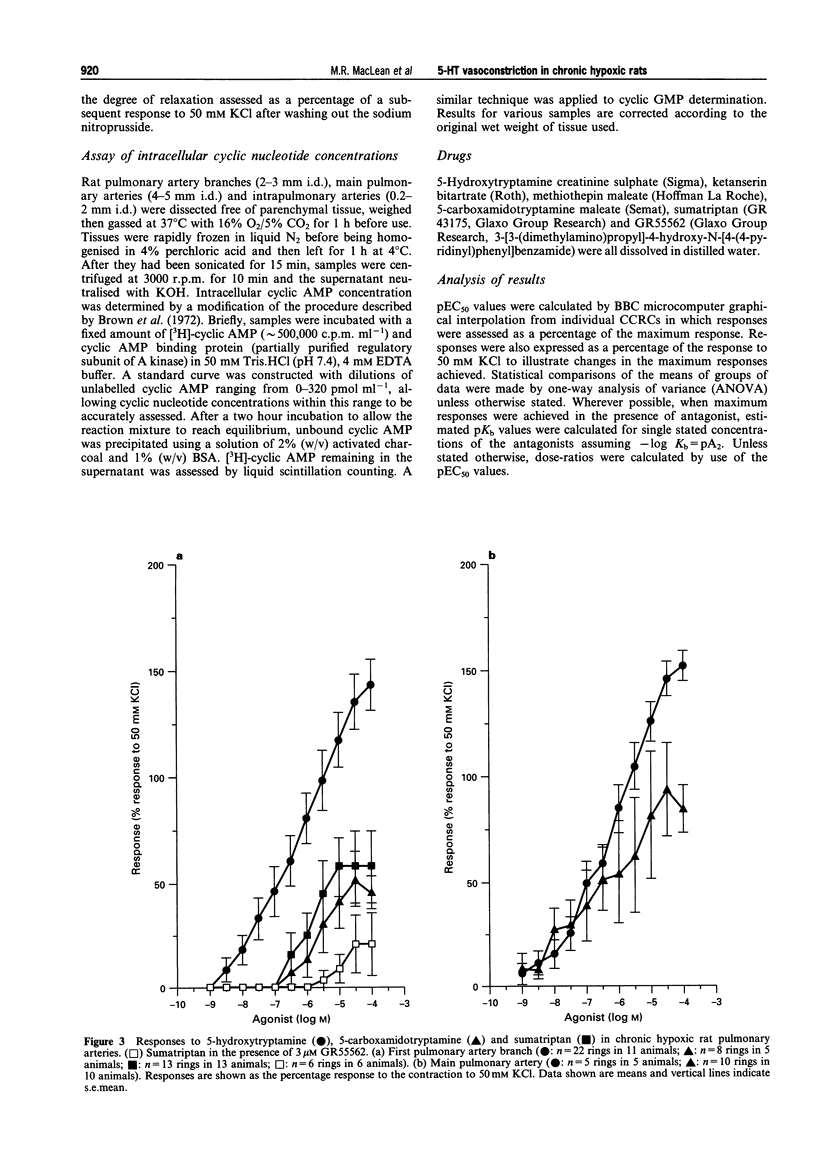
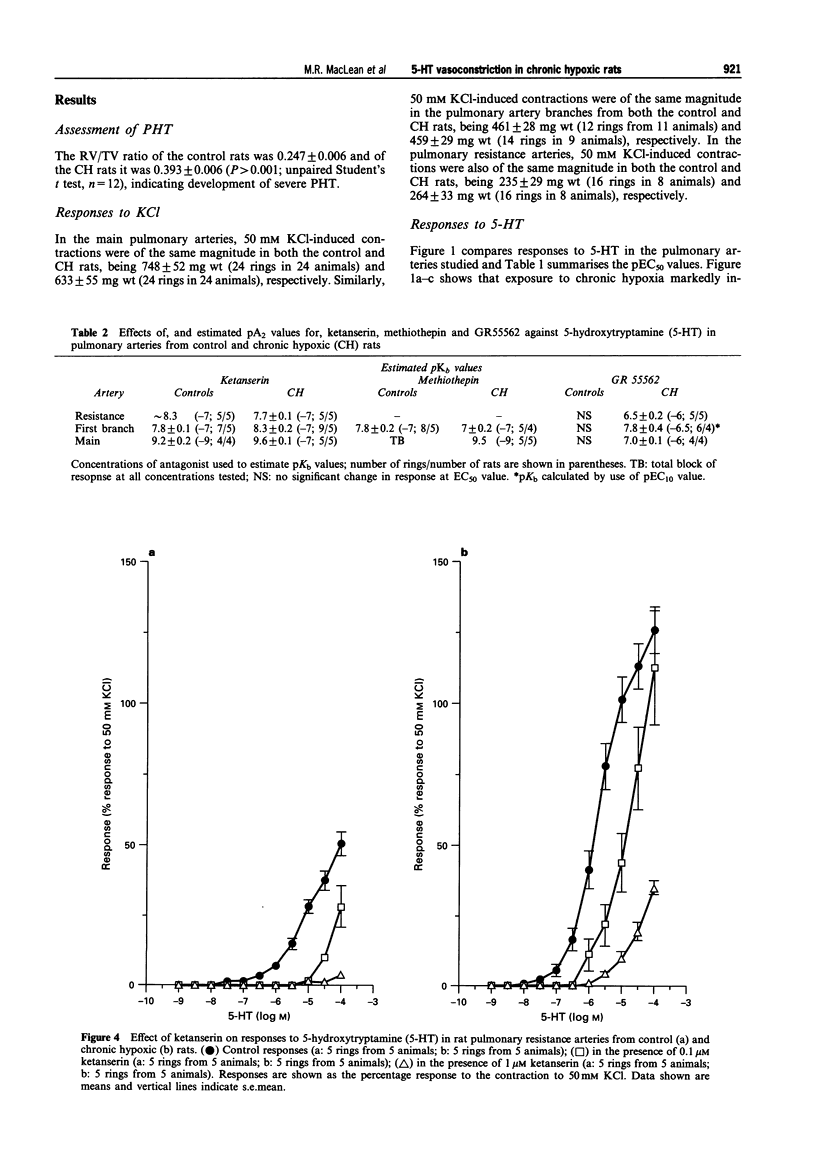
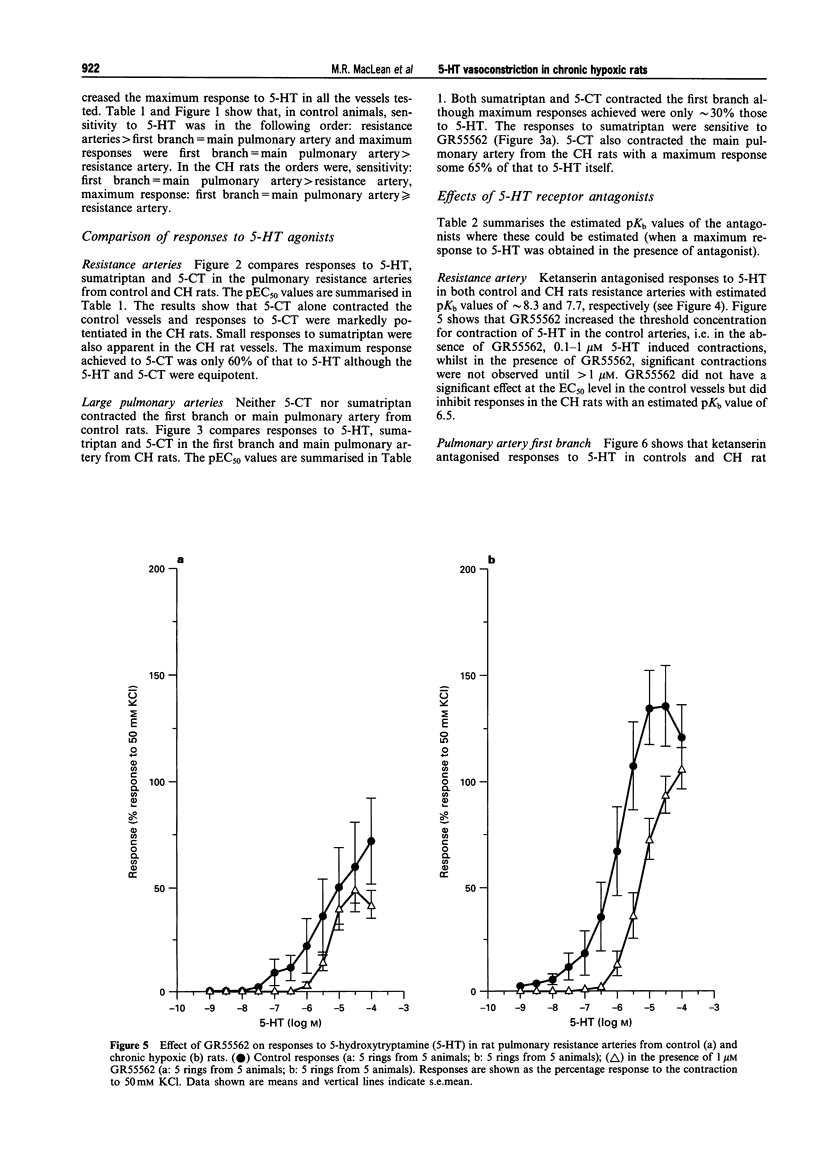
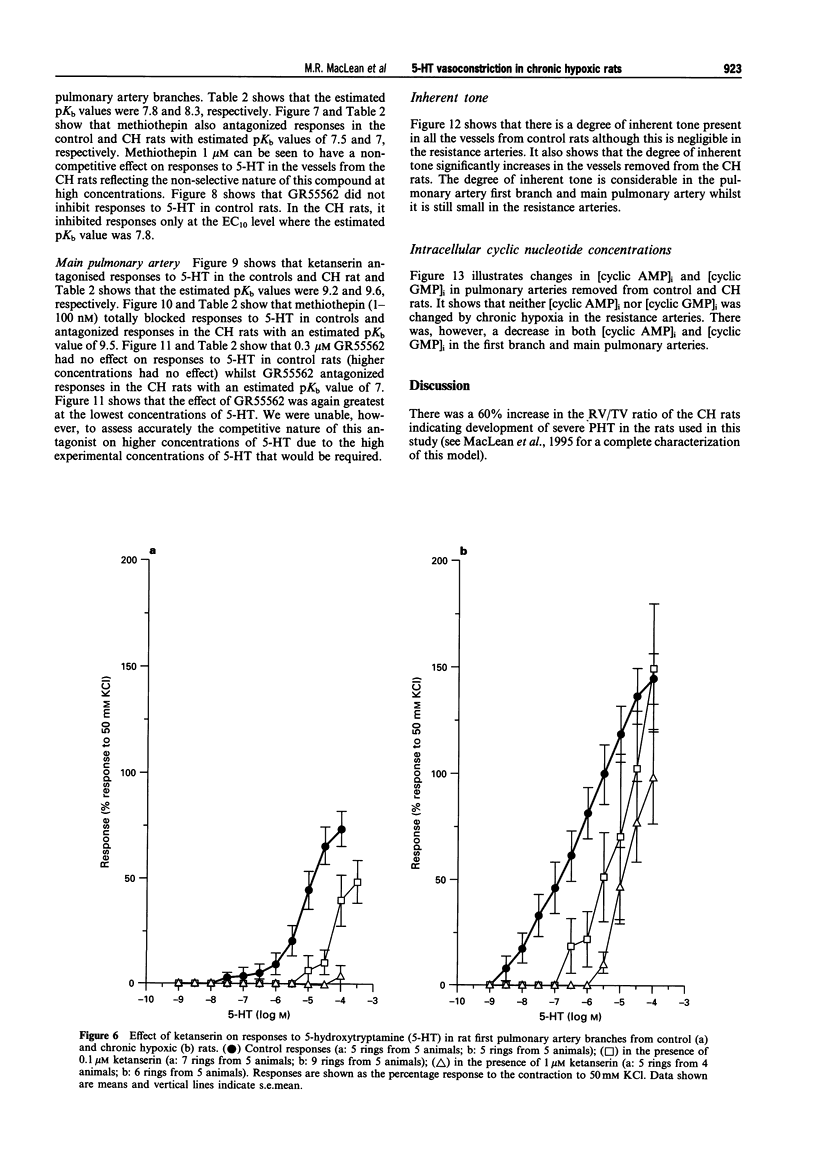
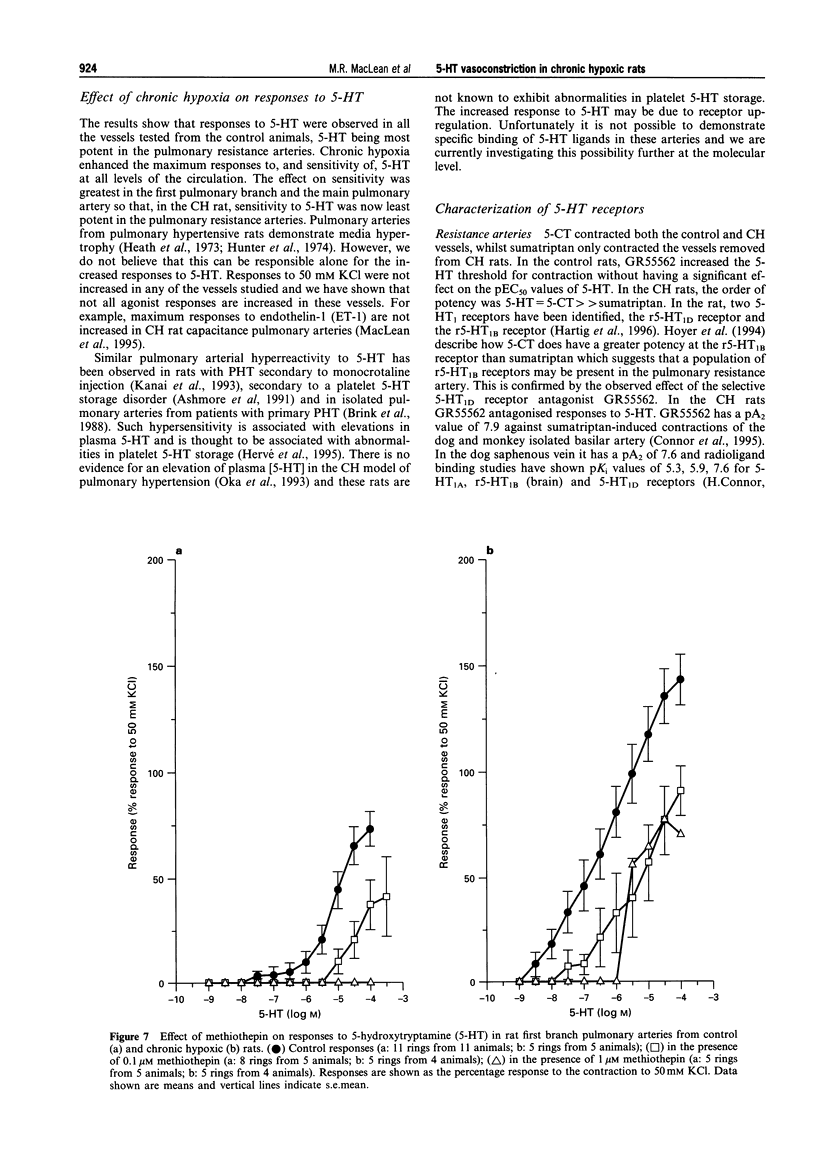
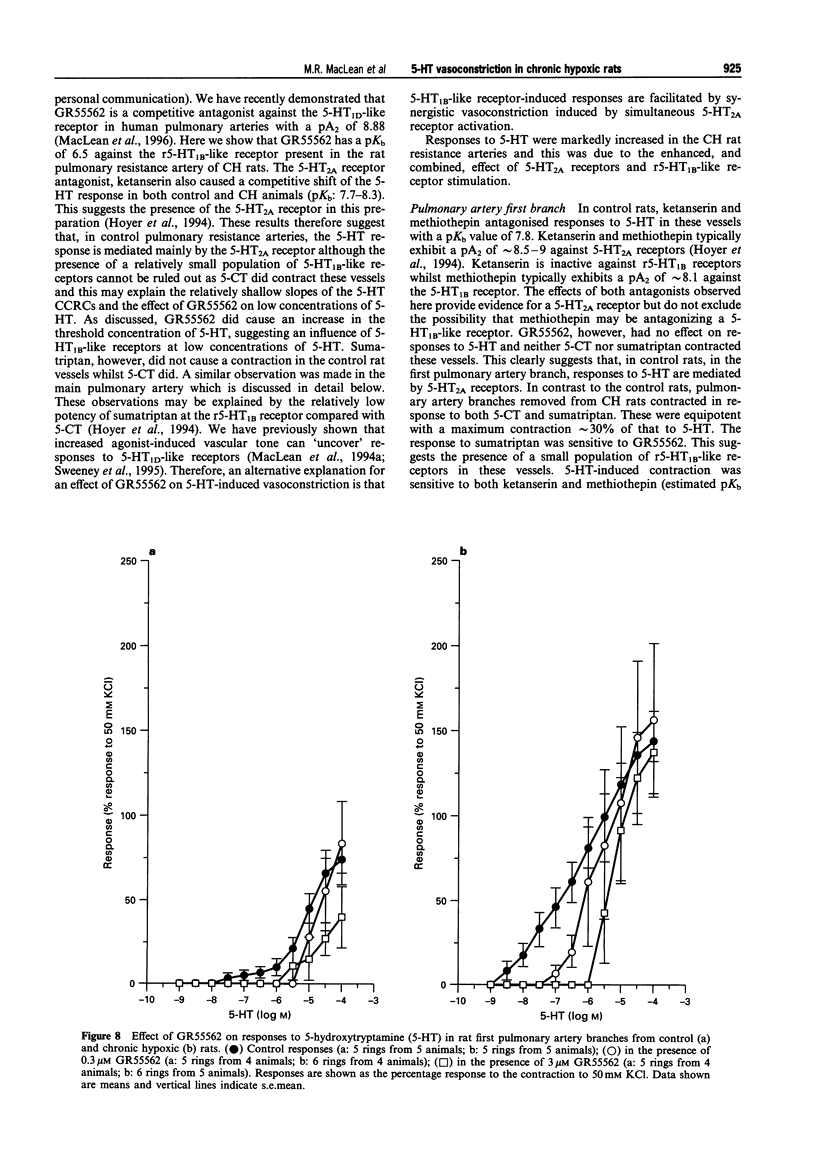
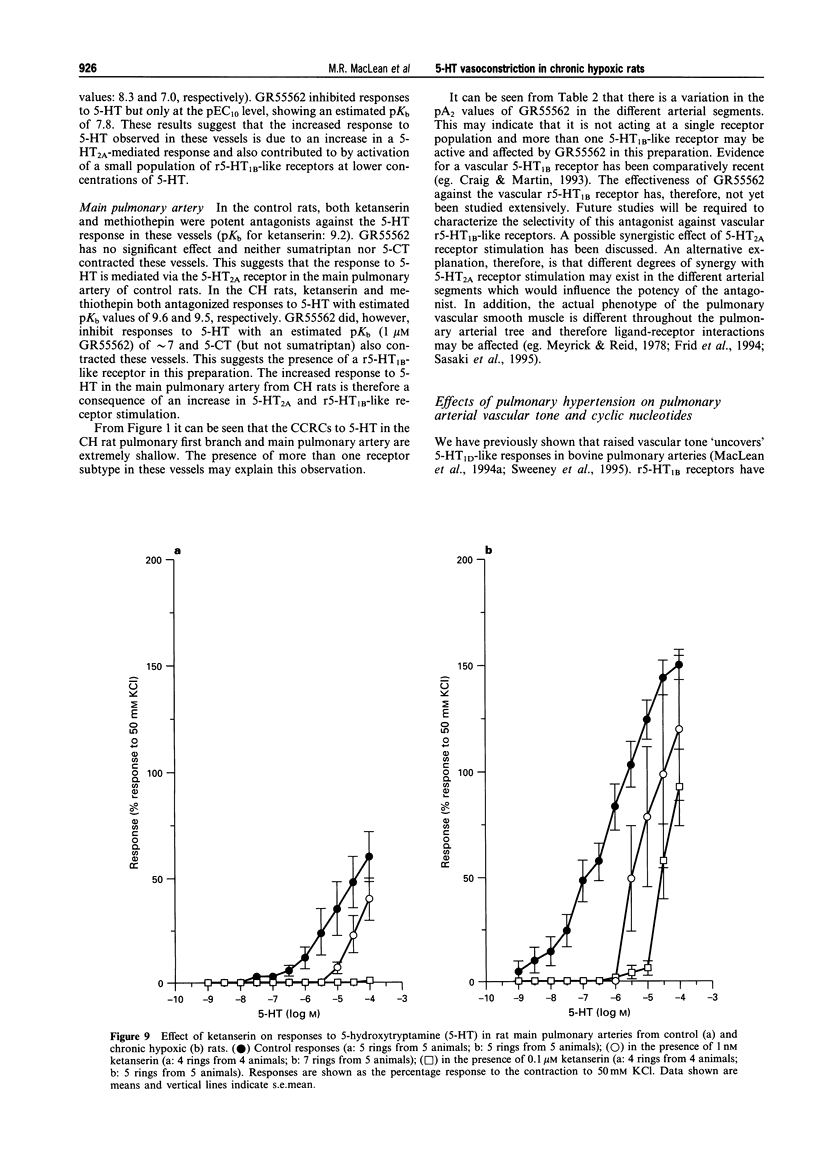
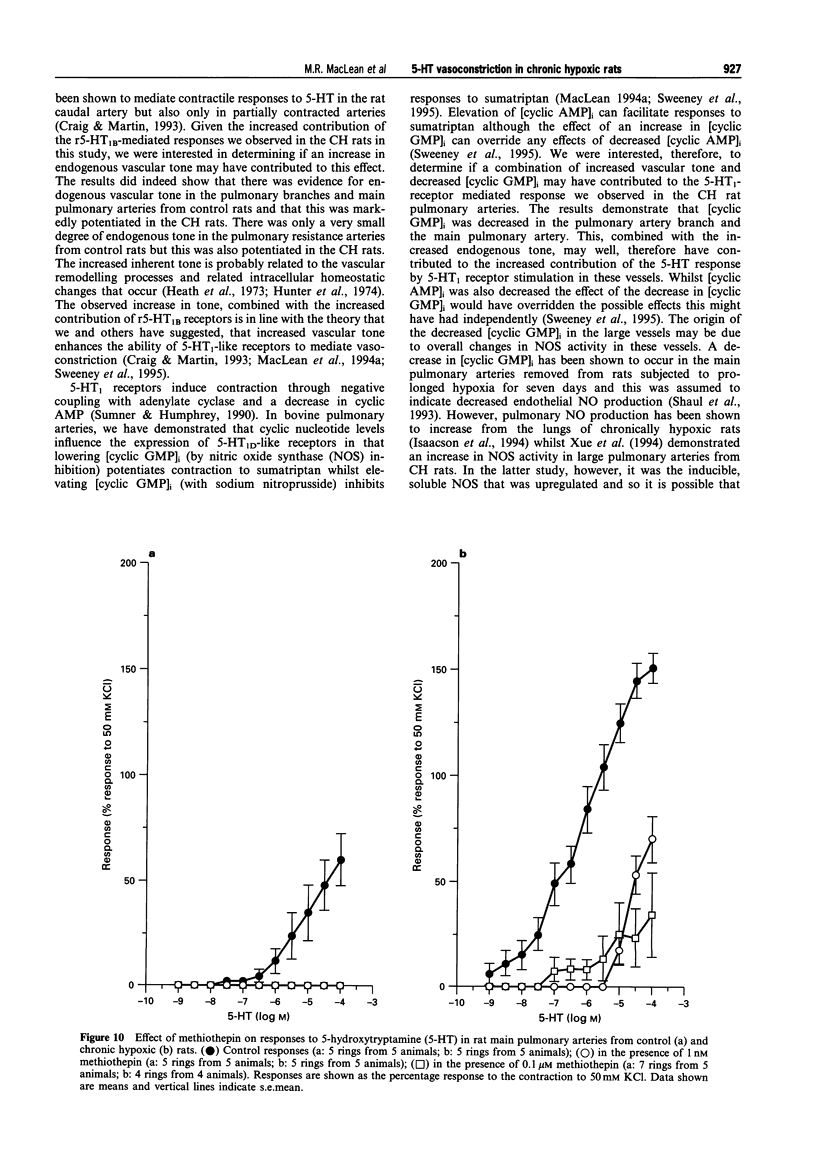
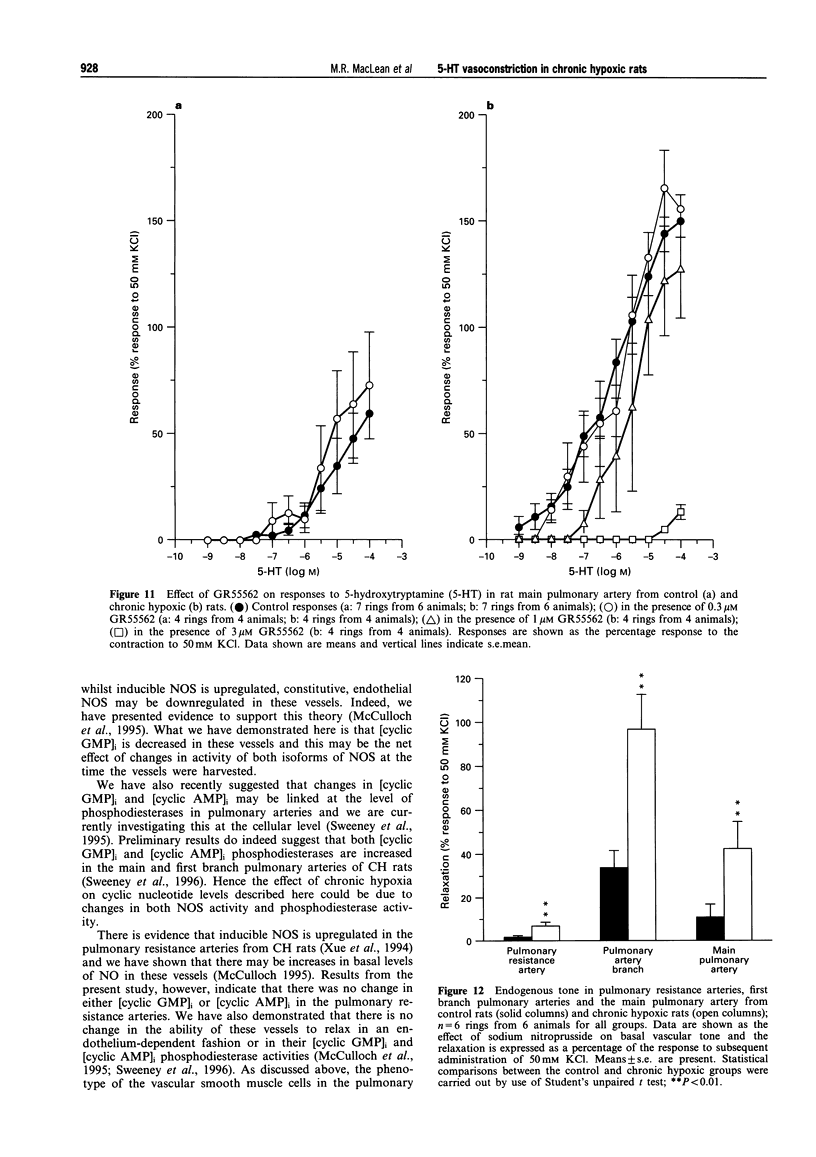
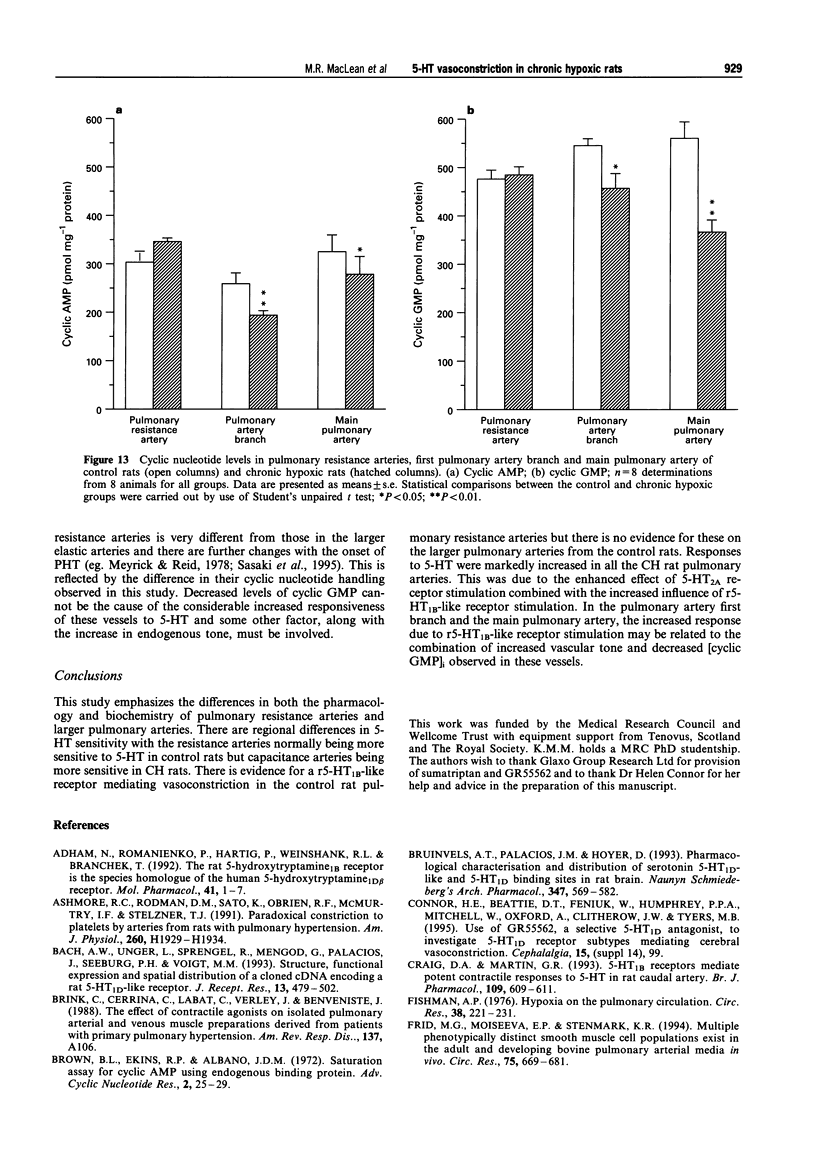
1. We investigated 5-hydroxytryptamine (5-HT)-receptor mediated vasoconstriction in the main, first branch and resistance pulmonary arteries removed from control and pulmonary hypertensive rats. Contractile responses to 5-HT, 5-carboxamidotryptamine (5-CT, non-selective 5-HT1 agonist), and sumatriptan (5-HT1D-like receptor agonist) were studied. The effects of methiothepin (non-selective 5-HT1 + 2-receptor antagonist) and ketanserin (5-HT2A receptor antagonist) and GR55562 (a novel selective 5-HT1D receptor antagonist) on 5-HT-mediated responses were also studied. Basal levels of adenosine 3':5'-cyclic monophosphate ([cyclic AMP]i) and guanosine 3':5'-cyclic monophosphate ([cyclic GMP]i) were determined and we assessed the degree of inherent tone in the vessels under study. 2. 5-HT was most potent in the resistance arteries. pEC50 values were 5.6 +/- 0.1, 5.3 +/- 0.1, 5.0 +/- 0.2 in the resistance arteries, pulmonary branch and main pulmonary artery, respectively (n = at least 5 from 5 animals). The sensitivity to, and maximum response of, 5-HT was increased in all the arteries removed from the chronic hypoxic (CH) rats. In CH rats the pEC50 values were 5.9 +/- 0.2, 6.3 +/- 0.2, 6.4 +/- 0.2 and the increase in the maximum response was 35%, 51% and 41% in the resistance arteries, pulmonary branch and main pulmonary artery, respectively. Sumatriptan did not contract any vessel from the control rats whilst 5-CT did contract the resistance arteries. In the CH rats, however, they both contracted the resistance arteries (responses to sumatriptan were small) (pEC50: 5-CT; 5.4 +/- 0.2) and the pulmonary artery branches (pEC50: sumatriptan, 5.4 +/- 0.2; 5-CT, 5.4 +/- 0.2). 5-CT also caused a contraction in the main pulmonary artery (pEC50: 6.0 +/- 0.3). 3. Ketanserin (1 nM-1 microM) caused a competitive antagonism of the 5-HT response in all vessels tested. In control rats, the estimated pKb values for ketanserin in resistance arteries, pulmonary branches and main pulmonary artery were 8.3, 7.8 and 9.2, respectively. Methiothepin (1 nM-1 microM) inhibited responses to 5-HT in the first branch (estimated pKb value: 7.8) and main pulmonary artery. In CH rats, the estimated pKb values for ketanserin in resistance arteries, pulmonary branches and main pulmonary artery were 7.7, 8.3 and 9.6, respectively. Methiothepin also inhibited contractions to 5-HT in the pulmonary artery branch and main pulmonary artery with estimated pKb values of 7 and 9.5, respectively. In control animals, GR55562 had no effect on responses to 5-HT in any of the vessels tested. In the CH rats the estimated pKb values for GR55562 were 6.5, 7.8 and 7.0 in the pulmonary resistance arteries, first branch and main pulmonary artery, respectively. 4. Large pulmonary arteries from controls demonstrated inherent tone and this was increased three fold in the CH rats. The resistance arteries from controls demonstrated little inherent tone though this was enhanced in those from the CH rats. 5. [Cyclic AMP]i was 259 +/- 23 pmol mg-1 protein in the pulmonary artery branches removed from control rats and decreased to 192 +/- 11 pml mg-1 protein in the CH rats (P < 0.01, n = 8). [Cyclic GMP]i also decreased in the pulmonary artery branches (from 550 +/- 15, control to 462 +/- 31 pmol mg-1 protein in CH vessels, n = 8, P < 0.01) and in the main pulmonary arteries (from 566 +/- 33, control to 370 +/- 25 pmol mg-1 protein in CH vessels, n = 8, P < 0.001). No changes in either [cyclic AMP]i or [cyclic GMP]i were observed in the resistance arteries. 6. The results suggest that the increased vasoconstrictor response to 5-HT in CH rat pulmonary arteries is due to an increase in 5-HT2A-receptor mediated contraction combined with an increase in r5-HT1B-like receptor-mediated contraction. An increase in vascular tone and decreased levels of [cyclic GMP]i in the large pulmonary arteries may contribute to the observed increase in activity of r5-HT1B-like receptor
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adham N., Romanienko P., Hartig P., Weinshank R. L., Branchek T. The rat 5-hydroxytryptamine1B receptor is the species homologue of the human 5-hydroxytryptamine1D beta receptor. Mol Pharmacol. 1992 Jan;41(1):1–7. [PubMed] [Google Scholar]
- Austin C. E., Foreman J. C. The effect of platelet-activating factor on the responsiveness of the human nasal airway. Br J Pharmacol. 1993 Sep;110(1):113–118. doi: 10.1111/j.1476-5381.1993.tb13779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach A. W., Unger L., Sprengel R., Mengod G., Palacios J., Seeburg P. H., Voigt M. M. Structure functional expression and spatial distribution of a cloned cDNA encoding a rat 5-HT1D-like receptor. J Recept Res. 1993;13(1-4):479–502. doi: 10.3109/10799899309073674. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Ekins R. P., Albano J. D. Saturation assay for cyclic AMP using endogenous binding protein. Adv Cyclic Nucleotide Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Bruinvels A. T., Palacios J. M., Hoyer D. Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1993 Jun;347(6):569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- Craig D. A., Martin G. R. 5-HT1B receptors mediate potent contractile responses to 5-HT in rat caudal artery. Br J Pharmacol. 1993 Jul;109(3):609–611. doi: 10.1111/j.1476-5381.1993.tb13615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman A. P. Hypoxia on the pulmonary circulation. How and where it acts. Circ Res. 1976 Apr;38(4):221–231. doi: 10.1161/01.res.38.4.221. [DOI] [PubMed] [Google Scholar]
- Frid M. G., Moiseeva E. P., Stenmark K. R. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res. 1994 Oct;75(4):669–681. doi: 10.1161/01.res.75.4.669. [DOI] [PubMed] [Google Scholar]
- Hartig P. R., Branchek T. A., Weinshank R. L. A subfamily of 5-HT1D receptor genes. Trends Pharmacol Sci. 1992 Apr;13(4):152–159. doi: 10.1016/0165-6147(92)90053-9. [DOI] [PubMed] [Google Scholar]
- Hartig P. R., Hoyer D., Humphrey P. P., Martin G. R. Alignment of receptor nomenclature with the human genome: classification of 5-HT1B and 5-HT1D receptor subtypes. Trends Pharmacol Sci. 1996 Mar;17(3):103–105. doi: 10.1016/0165-6147(96)30002-3. [DOI] [PubMed] [Google Scholar]
- Heath D., Edwards C., Winson M., Smith P. Effects on the right ventricle, pulmonary vasculature, and carotid bodies of the rat of exposure to, and recovery from, simulated high altitude. Thorax. 1973 Jan;28(1):24–28. doi: 10.1136/thx.28.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K., Titeler M. Detection and characterization of the serotonin 5-HT 1D receptor in rat and human brain. J Neurochem. 1988 May;50(5):1624–1631. doi: 10.1111/j.1471-4159.1988.tb03052.x. [DOI] [PubMed] [Google Scholar]
- Hervé P., Launay J. M., Scrobohaci M. L., Brenot F., Simonneau G., Petitpretz P., Poubeau P., Cerrina J., Duroux P., Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995 Sep;99(3):249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994 Jun;46(2):157–203. [PubMed] [Google Scholar]
- Humphrey P. P., Feniuk W., Perren M. J., Connor H. E., Oxford A. W., Coates L. H., Butina D. GR43175, a selective agonist for the 5-HT1-like receptor in dog isolated saphenous vein. Br J Pharmacol. 1988 Aug;94(4):1123–1132. doi: 10.1111/j.1476-5381.1988.tb11630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C., Barer G. R., Shaw J. W., Clegg E. J. Growth of the heart and lungs in hypoxic rodents: a model of human hypoxic disease. Clin Sci Mol Med. 1974 Mar;46(3):375–391. doi: 10.1042/cs0460375. [DOI] [PubMed] [Google Scholar]
- Isaacson T. C., Hampl V., Weir E. K., Nelson D. P., Archer S. L. Increased endothelium-derived NO in hypertensive pulmonary circulation of chronically hypoxic rats. J Appl Physiol (1985) 1994 Feb;76(2):933–940. doi: 10.1152/jappl.1994.76.2.933. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Georgieff M. K. Pulmonary neuroendocrine cells. Their secretory products and their potential roles in health and chronic lung disease in infancy. Am Rev Respir Dis. 1989 Dec;140(6):1807–1812. doi: 10.1164/ajrccm/140.6.1807. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Hori S., Tanaka T., Yasuoka M., Watanabe K., Aikawa N., Hosoda Y. Role of 5-hydroxytryptamine in the progression of monocrotaline induced pulmonary hypertension in rats. Cardiovasc Res. 1993 Sep;27(9):1619–1623. doi: 10.1093/cvr/27.9.1619. [DOI] [PubMed] [Google Scholar]
- MacLean M. R., Clayton R. A., Hillis S. W., McIntyre P. D., Peacock A. J., Templeton A. G. 5-HT1-receptor-mediated vasoconstriction in bovine isolated pulmonary arteries: influences of vascular endothelium and tone. Pulm Pharmacol. 1994 Feb;7(1):65–72. doi: 10.1006/pulp.1994.1007. [DOI] [PubMed] [Google Scholar]
- MacLean M. R., McCulloch K. M., Baird M. Effects of pulmonary hypertension on vasoconstrictor responses to endothelin-1 and sarafotoxin S6C and on inherent tone in rat pulmonary arteries. J Cardiovasc Pharmacol. 1995 Nov;26(5):822–830. doi: 10.1097/00005344-199511000-00020. [DOI] [PubMed] [Google Scholar]
- MacLean M. R., McCulloch K. M., Baird M. Endothelin ETA- and ETB-receptor-mediated vasoconstriction in rat pulmonary arteries and arterioles. J Cardiovasc Pharmacol. 1994 May;23(5):838–845. doi: 10.1097/00005344-199405000-00022. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest. 1978 Feb;38(2):188–200. [PubMed] [Google Scholar]
- Oka M., Hasunuma K., Webb S. A., Stelzner T. J., Rodman D. M., McMurtry I. F. EDRF suppresses an unidentified vasoconstrictor mechanism in hypertensive rat lungs. Am J Physiol. 1993 Jun;264(6 Pt 1):L587–L597. doi: 10.1152/ajplung.1993.264.6.L587. [DOI] [PubMed] [Google Scholar]
- Parsons A. A., Whalley E. T. Evidence for the presence of 5-HT1-like receptors in rabbit isolated basilar arteries. Eur J Pharmacol. 1989 Dec 19;174(2-3):189–196. doi: 10.1016/0014-2999(89)90311-7. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., McCarthy B. G. Sumatriptan (GR 43175) interacts selectively with 5-HT1B and 5-HT1D binding sites. Eur J Pharmacol. 1989 Apr 12;163(1):133–136. doi: 10.1016/0014-2999(89)90406-8. [DOI] [PubMed] [Google Scholar]
- Pittman R. N., Duling B. R. Oxygen sensitivity of vascular smooth muscle. I. In vitro studies. Microvasc Res. 1973 Sep;6(2):202–211. doi: 10.1016/0026-2862(73)90020-4. [DOI] [PubMed] [Google Scholar]
- Reneman R. S., van der Starre P. J. Serotonin and acute cardiovascular disorders. Cardiovasc Drugs Ther. 1990 Jan;4 (Suppl 1):19–25. doi: 10.1007/BF00053422. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Kobayashi N., Dambara T., Kira S., Sakai T. Structural organization of pulmonary arteries in the rat lung. Anat Embryol (Berl) 1995 Jun;191(6):477–489. doi: 10.1007/BF00186738. [DOI] [PubMed] [Google Scholar]
- Saxena P. R., Villalón C. M. Cardiovascular effects of serotonin agonists and antagonists. J Cardiovasc Pharmacol. 1990;15 (Suppl 7):S17–S34. [PubMed] [Google Scholar]
- Shaul P. W., Wells L. B., Horning K. M. Acute and prolonged hypoxia attenuate endothelial nitric oxide production in rat pulmonary arteries by different mechanisms. J Cardiovasc Pharmacol. 1993 Dec;22(6):819–827. doi: 10.1097/00005344-199312000-00007. [DOI] [PubMed] [Google Scholar]
- Staub N. C. Site of hypoxic pulmonary vasoconstriction. Chest. 1985 Oct;88(4 Suppl):240S–245S. doi: 10.1378/chest.88.4_supplement.240s. [DOI] [PubMed] [Google Scholar]
- Sumner M. J., Humphrey P. P. Sumatriptan (GR43175) inhibits cyclic-AMP accumulation in dog isolated saphenous vein. Br J Pharmacol. 1990 Feb;99(2):219–220. doi: 10.1111/j.1476-5381.1990.tb14682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney G., Templeton A., Clayton R. A., Baird M., Sheridan S., Johnston E. D., MacLean M. R. Contractile responses to sumatriptan in isolated bovine pulmonary artery rings: relationship to tone and cyclic nucleotide levels. J Cardiovasc Pharmacol. 1995 Nov;26(5):751–760. doi: 10.1097/00005344-199511000-00012. [DOI] [PubMed] [Google Scholar]
- Voigt M. M., Laurie D. J., Seeburg P. H., Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J. 1991 Dec;10(13):4017–4023. doi: 10.1002/j.1460-2075.1991.tb04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanstall J. C., O'Donnell S. R. Endothelin and 5-hydroxytryptamine on rat pulmonary artery in pulmonary hypertension. Eur J Pharmacol. 1990 Feb 6;176(2):159–168. doi: 10.1016/0014-2999(90)90524-a. [DOI] [PubMed] [Google Scholar]
- Wanstall J. C., O'Donnell S. R., Kay C. S. Increased relaxation by felodipine on pulmonary artery from rats with monocrotaline-induced pulmonary hypertension does not reflect functional impairment of the endothelium. Pulm Pharmacol. 1991;4(1):60–66. doi: 10.1016/0952-0600(91)90041-z. [DOI] [PubMed] [Google Scholar]
- Xue C., Rengasamy A., Le Cras T. D., Koberna P. A., Dailey G. C., Johns R. A. Distribution of NOS in normoxic vs. hypoxic rat lung: upregulation of NOS by chronic hypoxia. Am J Physiol. 1994 Dec;267(6 Pt 1):L667–L678. doi: 10.1152/ajplung.1994.267.6.L667. [DOI] [PubMed] [Google Scholar]


