Abstract
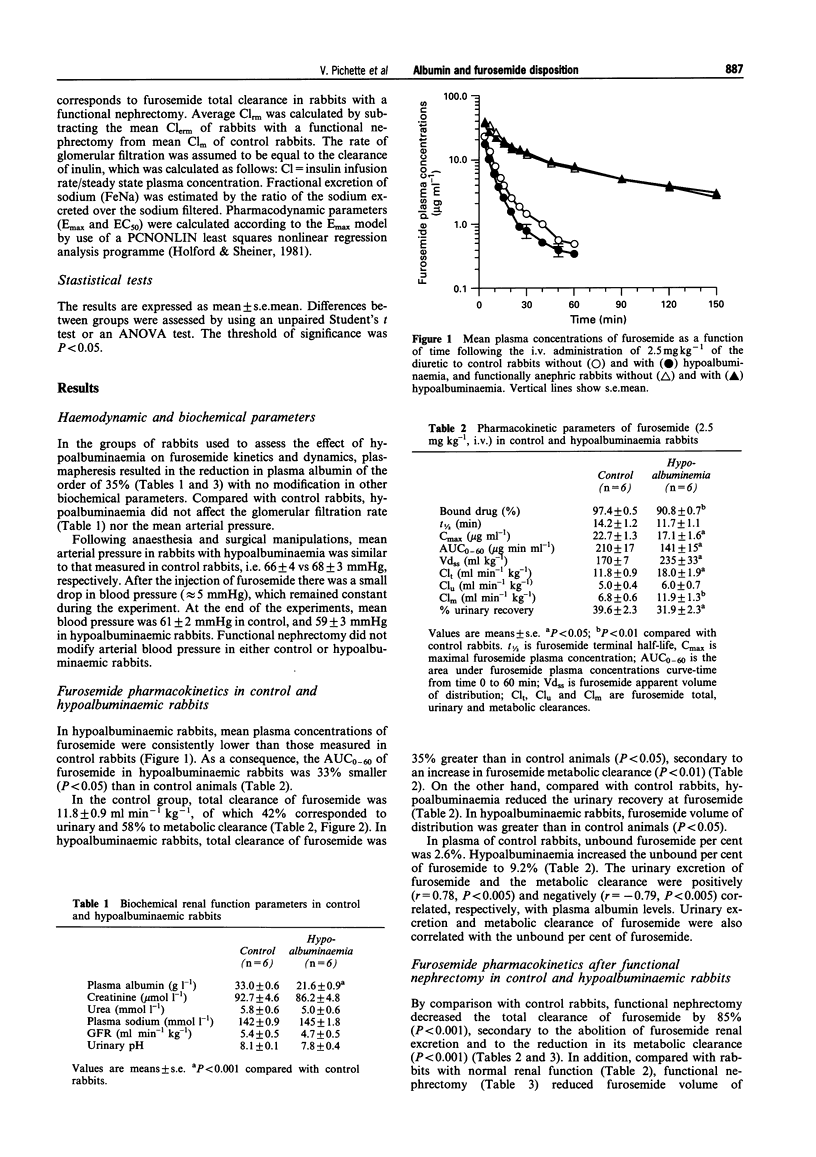
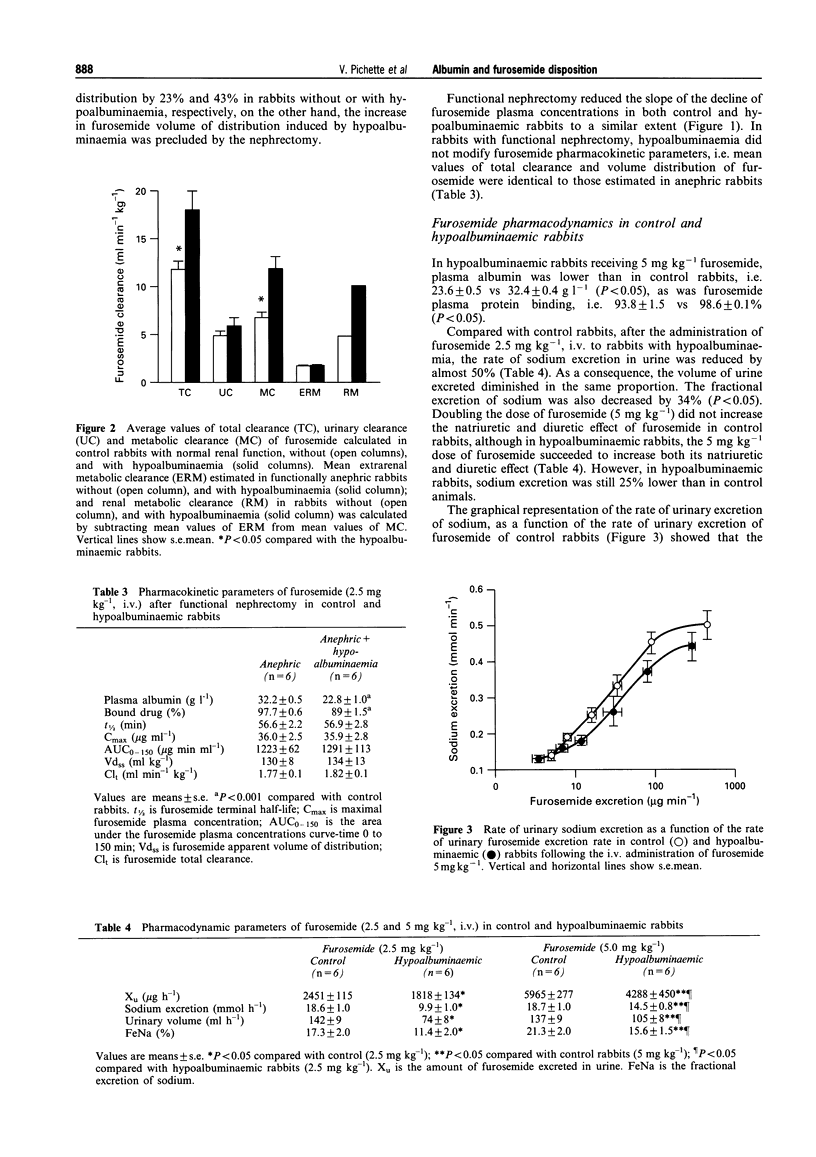
1. The present study aimed to investigate the influence of hypoalbuminaemia on the pharmacokinetics and pharmacodynamics of furosemide. Hypoalbuminaemia was produced by repeated plasmapheresis, to attain plasma albumin concentrations of 21.6 +/- 0.9 g l-1, compared with 33.0 +/- 0.6 g l-1 in controls (P < 0.001). The per cent of bound furosemide in hypoalbuminaemic rabbits (90.8 +/- 0.7%) was lower than that in control rabbits (97.4 +/- 0.5%, P < 0.001). The kinetics of intravenous furosemide (2.5 mg kg-1) were studied in control (n = 6) and hypoalbuminaemic rabbits (n = 6). 2. To assess the effect of hypoalbuminaemia on extrarenal clearance of furosemide, functional anephria was induced by ligating the renal pedicles of 12 rabbits, which were segregated in two groups, with and without hypoalbuminaemia. 3. In the control group, total, urinary and metabolic clearances of furosemide were 11.8 +/- 1.0, 5.0 +/- 0.4 and 6.8 +/- 0.6 ml min-1 kg-1, respectively. Compared with control rabbits, in hypoalbuminaemic rabbits, total clearance of furosemide increased by 40% (P < 0.001), result of the enhancement of furosemide metabolic clearance (C1m) from 5 to 10 ml min-1 kg-1 (P < 0.01). In hypoalbuminaemic rabbits, urinary excretion of furosemide was reduced by 26% (2451 +/- 115 vs 1818 +/- 134 micrograms h-1, P < 0.01). In anephric rabbits, furosemide total clearance was 1.77 +/- 0.12 ml min-1 kg-1, value not affected by hypoalbuminaemia, confirming that the increase in C1m induced by hypoalbuminaemia occurs in the kidneys. 4. Compared with controls, in hypoalbuminaemic rabbits, the rate of urinary excretion (142 +/- 9 vs 74 +/- 8 ml h-1, P < 0.001) and the rate of excretion of sodium (18.6 +/- 0.9 vs 9.9 +/- 0.9 mmol h-1, P < 0.001) were very much reduced. However, the dose-response curves were not different. 5. In conclusion, hypoalbuminaemia is associated with an increase in renal metabolic clearance of furosemide, possibly because of the increase in furosemide unbound concentration, and a decrease in the diuretic/natriuretic effect of furosemide, secondary to a reduction in furosemide tubular secretion. Thus, albumin facilitates the renal secretion of organic anions but not their metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besseghir K., Mosig D., Roch-Ramel F. Facilitation by serum albumin of renal tubular secretion of organic anions. Am J Physiol. 1989 Mar;256(3 Pt 2):F475–F484. doi: 10.1152/ajprenal.1989.256.3.F475. [DOI] [PubMed] [Google Scholar]
- Depner T. A., Sanaka T., Stanfel L. A. Suppression of para-aminohippurate transport in the isolated perfused kidney by an inhibitor of protein binding in uremia. Am J Kidney Dis. 1984 Jan;3(4):280–286. doi: 10.1016/s0272-6386(84)80046-3. [DOI] [PubMed] [Google Scholar]
- Green T. P., Mirkin B. L. Furosemide disposition in normal and proteinuric rats: urinary drug-protein binding as a determinant of drug excretion. J Pharmacol Exp Ther. 1981 Jul;218(1):122–127. [PubMed] [Google Scholar]
- Hammarlund-Udenaes M., Benet L. Z. Furosemide pharmacokinetics and pharmacodynamics in health and disease--an update. J Pharmacokinet Biopharm. 1989 Feb;17(1):1–46. doi: 10.1007/BF01059086. [DOI] [PubMed] [Google Scholar]
- Holford N. H., Sheiner L. B. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 1981 Nov-Dec;6(6):429–453. doi: 10.2165/00003088-198106060-00002. [DOI] [PubMed] [Google Scholar]
- Homeida M., Roberts C., Branch R. A. Influence of probenecid and spironolactone on furosemide kinetics and dynamics in man. Clin Pharmacol Ther. 1977 Oct;22(4):402–409. doi: 10.1002/cpt1977224402. [DOI] [PubMed] [Google Scholar]
- Homsy W., Marleau S., du Souich P. Furosemide dynamics in conscious rabbits: modulation by angiotensin II. Cardiovasc Drugs Ther. 1995 Apr;9(2):311–317. doi: 10.1007/BF00878676. [DOI] [PubMed] [Google Scholar]
- Inoue M., Koyama H., Nagase S., Morino Y. Renal secretion of phenolsulfonphthalein: analysis of its vectorial transport in normal and mutant analbuminemic rats. J Lab Clin Med. 1985 Apr;105(4):484–488. [PubMed] [Google Scholar]
- Inoue M., Okajima K., Itoh K., Ando Y., Watanabe N., Yasaka T., Nagase S., Morino Y. Mechanism of furosemide resistance in analbuminemic rats and hypoalbuminemic patients. Kidney Int. 1987 Aug;32(2):198–203. doi: 10.1038/ki.1987.192. [DOI] [PubMed] [Google Scholar]
- Inoue M., Okajima K., Nagase S., Morino Y. Plasma clearance of sulfobromophthalein and its interaction with hepatic binding proteins in normal and analbuminemic rats: is plasma albumin essential for vectorial transport of organic anions in the liver? Proc Natl Acad Sci U S A. 1983 Dec;80(24):7654–7658. doi: 10.1073/pnas.80.24.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E., Hoppe-Seyler G., Schollmeyer P. Disposition and diuretic effect of furosemide in the nephrotic syndrome. Clin Pharmacol Ther. 1982 Oct;32(4):442–449. doi: 10.1038/clpt.1982.187. [DOI] [PubMed] [Google Scholar]
- Lambert C., Caillé G., du Souich P. Nonrenal clearance of furosemide as a cause of diuretic response variability in the rat. J Pharmacol Exp Ther. 1982 Jul;222(1):232–236. [PubMed] [Google Scholar]
- Odlind B. Relationship between tubular secretion of furosemide and its saluretic effect. J Pharmacol Exp Ther. 1979 Mar;208(3):515–521. [PubMed] [Google Scholar]
- Okajima K., Inoue M., Itoh K., Nagase S., Morino Y. Role of plasma albumin in renal elimination of a mercapturic acid. Analyses in normal and mutant analbuminemic rats. Eur J Biochem. 1985 Jul 1;150(1):195–199. doi: 10.1111/j.1432-1033.1985.tb09007.x. [DOI] [PubMed] [Google Scholar]
- Ponto L. L., Schoenwald R. D. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part I). Clin Pharmacokinet. 1990 May;18(5):381–408. doi: 10.2165/00003088-199018050-00004. [DOI] [PubMed] [Google Scholar]
- Ponto L. L., Schoenwald R. D. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part II). Clin Pharmacokinet. 1990 Jun;18(6):460–471. doi: 10.2165/00003088-199018060-00003. [DOI] [PubMed] [Google Scholar]
- SCHREINER G. E. Determination of inulin by means of resorcinol. Proc Soc Exp Biol Med. 1950 May;74(1):117–120. doi: 10.3181/00379727-74-17827. [DOI] [PubMed] [Google Scholar]
- Schäli C., Roch-Ramel F. Transport and metabolism of [3H]morphine in isolated, nonperfused proximal tubular segments of the rabbit kidney. J Pharmacol Exp Ther. 1982 Dec;223(3):811–815. [PubMed] [Google Scholar]
- Smith D. E., Hyneck M. L., Berardi R. R., Port F. K. Urinary protein binding, kinetics, and dynamics of furosemide in nephrotic patients. J Pharm Sci. 1985 Jun;74(6):603–607. doi: 10.1002/jps.2600740604. [DOI] [PubMed] [Google Scholar]
- Sommers D. K., Meyer E. C., Moncrieff J. The influence of co-administered organic acids on the kinetics and dynamics of frusemide. Br J Clin Pharmacol. 1991 Oct;32(4):489–493. doi: 10.1111/j.1365-2125.1991.tb03936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J. A., Klotman P. E. Are we missing an epidemic of HIV-associated nephropathy? J Am Soc Nephrol. 1996 Jan;7(1):1–7. doi: 10.1681/ASN.V711. [DOI] [PubMed] [Google Scholar]
- Woodhall P. B., Tisher C. C., Simonton C. A., Robinson R. R. Relationship between para-aminohippurate secretion and cellular morphology in rabbit proximal tubules. J Clin Invest. 1978 May;61(5):1320–1329. doi: 10.1172/JCI109049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginneken C. A., Russel F. G. Saturable pharmacokinetics in the renal excretion of drugs. Clin Pharmacokinet. 1989 Jan;16(1):38–54. doi: 10.2165/00003088-198916010-00003. [DOI] [PubMed] [Google Scholar]


