Abstract
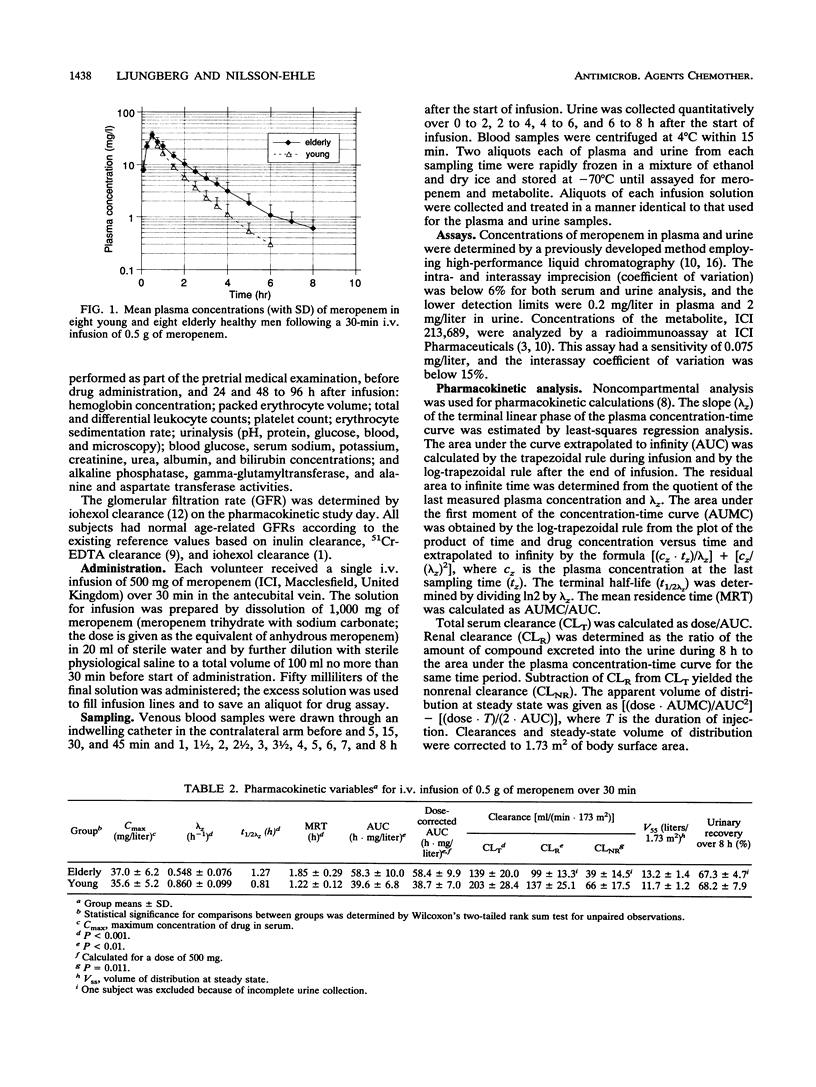
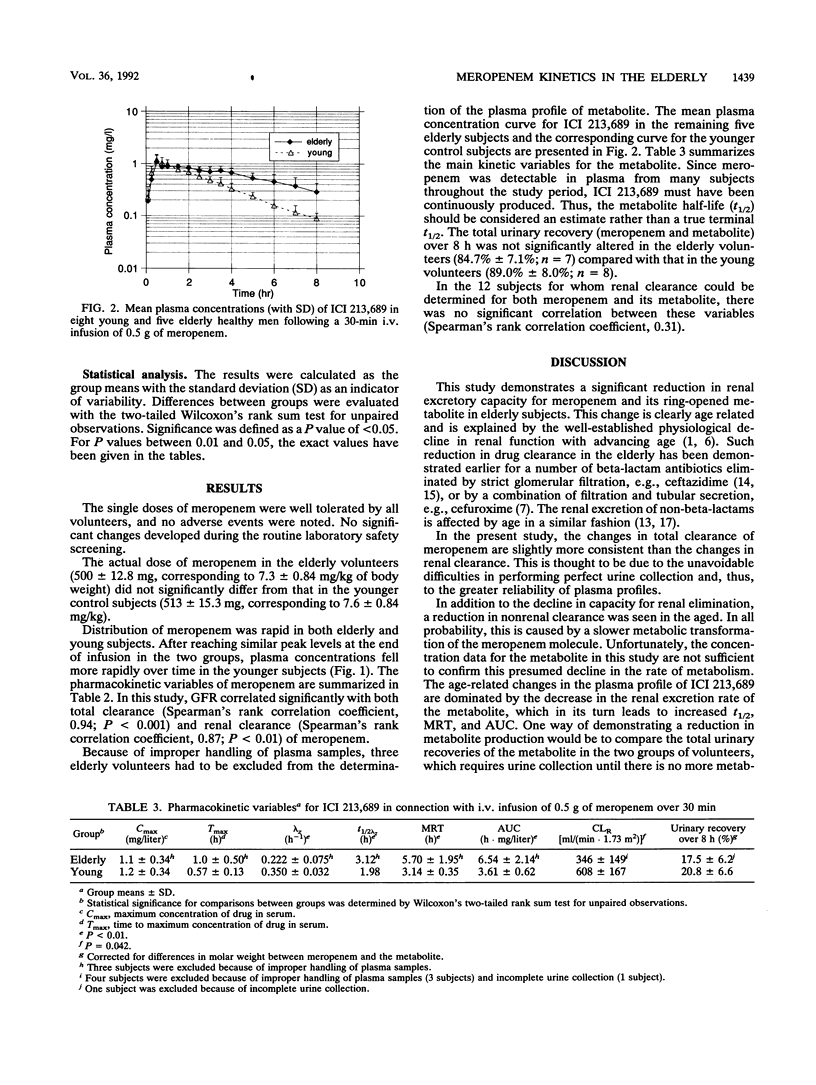
The pharmacokinetics of meropenem and its ring-opened metabolite (ICI 213,689) were investigated with eight young (20- to 34-year-old) and eight elderly (67- to 80-year-old) healthy male volunteers given single 30-min intravenous infusions of 500 mg of meropenem. All subjects had normal age-correlated glomerular function. The mean terminal half-life of meropenem was 1.27 h in the elderly subjects versus 0.81 h in the younger subjects (P less than 0.001). This and similar increases in mean residence time and area under the concentration-time curve were explained by a reduction in total [139 versus 203 ml/(min.1.73 m2); P less than 0.001], renal, and nonrenal clearances in subjects at advanced ages. The apparent volume of distribution and urinary recovery over 8 h were not significantly altered. With the metabolite, prolonged serum half-life and mean residence time, enlarged area under the concentration-time curve, and lower renal clearance but no significant changes in peak plasma concentration or urinary recovery were found in the elderly. The reduction in the renal excretion rate of meropenem and its metabolite corresponds to the age-associated physiological decline in renal function. The capacity to metabolize meropenem may also be slightly impaired in people at advanced ages. Dose reduction of meropenem should be considered for elderly patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax R. P., Bastain W., Featherstone A., Wilkinson D. M., Hutchison M., Haworth S. J. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):311–320. doi: 10.1093/jac/24.suppl_a.311. [DOI] [PubMed] [Google Scholar]
- Burman L. A., Nilsson-Ehle I., Hutchison M., Haworth S. J., Norrby S. R. Pharmacokinetics of meropenem and its metabolite ICI 213,689 in healthy subjects with known renal metabolism of imipenem. J Antimicrob Chemother. 1991 Feb;27(2):219–224. doi: 10.1093/jac/27.2.219. [DOI] [PubMed] [Google Scholar]
- Bäck S. E., Ljungberg B., Nilsson-Ehle I., Borgå O., Nilsson-Ehle P. Age dependence of renal function: clearance of iohexol and p-amino hippurate in healthy males. Scand J Clin Lab Invest. 1989 Nov;49(7):641–646. doi: 10.1080/00365518909091539. [DOI] [PubMed] [Google Scholar]
- Christensson B. A., Nilsson-Ehle I., Hutchison M., Haworth S. J., Oqvist B., Norrby S. R. Pharmacokinetics of meropenem in subjects with various degrees of renal impairment. Antimicrob Agents Chemother. 1992 Jul;36(7):1532–1537. doi: 10.1128/aac.36.7.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES D. F., SHOCK N. W. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950 May;29(5):496–507. doi: 10.1172/JCI102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J. G., Bax R. P., Munro J. F. The pharmacokinetics of cefuroxime in the elderly. J Antimicrob Chemother. 1980 Jul;6(4):543–549. doi: 10.1093/jac/6.4.543. [DOI] [PubMed] [Google Scholar]
- Granerus G., Aurell M. Reference values for 51Cr-EDTA clearance as a measure of glomerular filtration rate. Scand J Clin Lab Invest. 1981 Oct;41(6):611–616. doi: 10.3109/00365518109090505. [DOI] [PubMed] [Google Scholar]
- Harrison M. P., Moss S. R., Featherstone A., Fowkes A. G., Sanders A. M., Case D. E. The disposition and metabolism of meropenem in laboratory animals and man. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):265–277. doi: 10.1093/jac/24.suppl_a.265. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C. In-vitro studies of meropenem. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):9–29. doi: 10.1093/jac/24.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- Krutzén E., Bäck S. E., Nilsson-Ehle I., Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984 Dec;104(6):955–961. [PubMed] [Google Scholar]
- Ljungberg B., Nilsson-Ehle I. Advancing age and acute infection influence the kinetics of ceftazidime. Scand J Infect Dis. 1989;21(3):327–332. doi: 10.3109/00365548909035704. [DOI] [PubMed] [Google Scholar]
- Ljungberg B., Nilsson-Ehle I. Influence of age on the pharmacokinetics of ceftazidime in acutely ill, adult patients. Eur J Clin Pharmacol. 1988;34(2):173–178. doi: 10.1007/BF00614555. [DOI] [PubMed] [Google Scholar]
- Ljungberg B., Nilsson-Ehle I. Pharmacokinetics of antimicrobial agents in the elderly. Rev Infect Dis. 1987 Mar-Apr;9(2):250–264. doi: 10.1093/clinids/9.2.250. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle I., Hutchison M., Haworth S. J., Norrby S. R. Pharmacokinetics of meropenem compared to imipenem-cilastatin in young, healthy males. Eur J Clin Microbiol Infect Dis. 1991 Feb;10(2):85–88. doi: 10.1007/BF01964413. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle I., Ljungberg B. Quinolone disposition in the elderly. Practical implications. Drugs Aging. 1991 Jul-Aug;1(4):279–288. doi: 10.2165/00002512-199101040-00004. [DOI] [PubMed] [Google Scholar]
- Siersbaek-Nielsen K., Hansen J. M., Kampmann J., Kristensen M. Rapid evaluation of creatinine clearance. Lancet. 1971 May 29;1(7709):1133–1134. doi: 10.1016/s0140-6736(71)91873-3. [DOI] [PubMed] [Google Scholar]


