Abstract
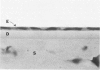
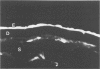
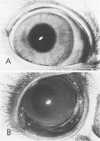
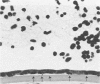
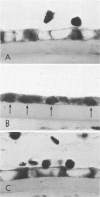
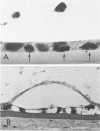
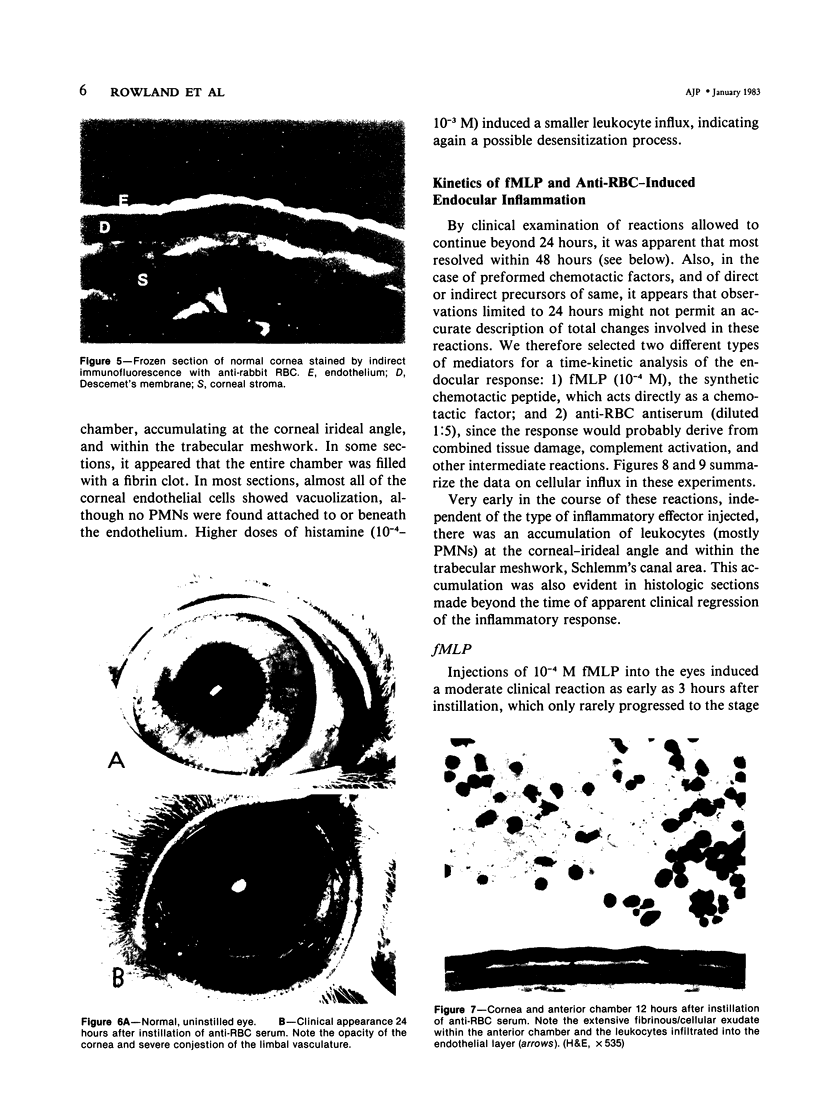
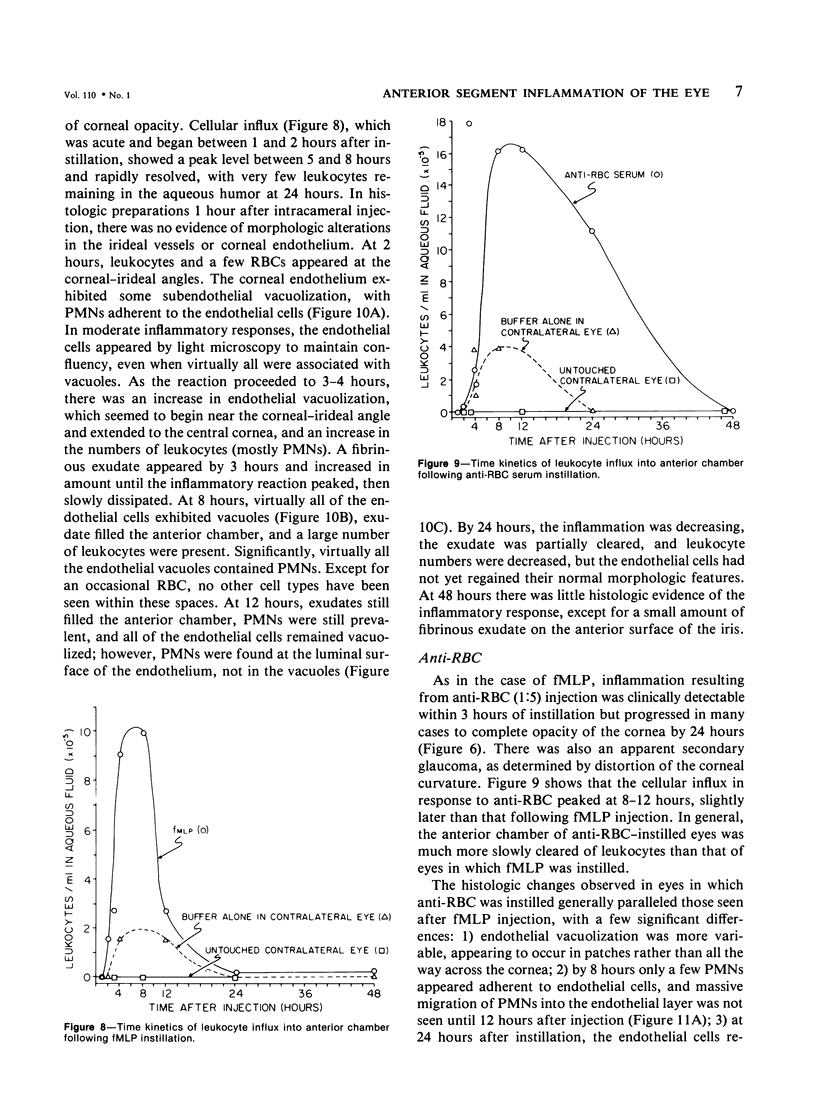
Although some investigations have demonstrated the ability of inflammatory mediators, including vasopermeability and chemotactic factors, to induce acute inflammatory reactions in vivo, little is known about the response of various elements of the anterior segment to the direct effects of inflammatory mediators. These studies were initiated to develop models for the investigation of inflammatory responses in this region of the eye. Acute inflammatory reactions were induced within the rabbit anterior chamber by intracameral injection of 50 microliters of various inflammatory mediators and were evaluated by clinical grade, leukocyte influx into the aqueous humor, and morphologic changes in the corneal endothelium. Peak responses were recorded following injection of 10(-4) M formyl-methionyl-leucyl-phenylalanine (fMLP); 5 ED50 C5fr; 0.5 mg/ml C5; undiluted anti-red blood cell (RBC) serum; and 10(-5) M histamine. The number of leukocytes per milliliter of aqueous humor induced by each mediator was quantitated by comparison with the number of leukocytes induced by buffer instillation into a separate group of rabbits (mediator-induced influx/buffer-induced influx). Comparisons were made 24 hours after instillation of mediators. The results of these studies were as follows: buffer alone, 1.0; fMLP, 3.1 C5fr, 61.0; C5, 8.7; anti-RBC, 91.0; and histamine, 24.0. Clinical grades correlated well with these ratios. In addition, differences were noted when the time kinetics of acute responses induced by two different mediators (10(-4) M fMLP, a synthetic preformed chemotactic factor; and a 1:5 dilution of anti-RBC, which binds to vascular and corneal endothelial cells) were directly compared over 48 hours. Responses induced with fMLP peaked between 5 and 8 hours and resolved rapidly, whereas anti-RBC-induced responses peaked between 8 and 12 hours and resolved very slowly. Histopathologic analysis indicated that both fMLP and anti-RBC induced a similar sequence of changes in the corneal endothelium. Within 2-3 hours after instillation of either mediator, the endothelial cells exhibited prominent vacuolization/retraction phenomena. At the peak of leukocyte influx PMNs filled these vacuoles, then migrated back into the aqueous humor within several hours. Normal morphologic features were recovered following clearance of leukocytes from the anterior chamber. We believe that these models will be useful in identifying the roles of individual mediators in acute and chronic endocular inflammation and in the injury of corneal endothelium.
Full text
PDF





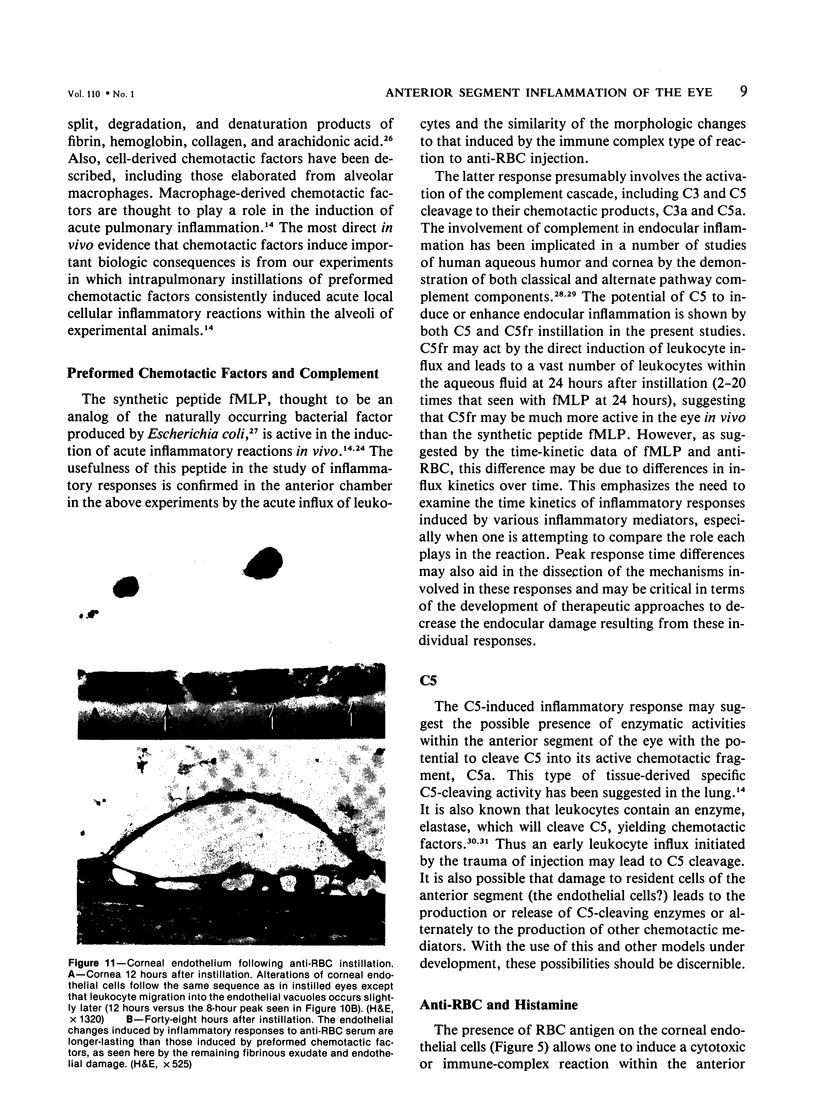



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson S. B., Howes E. L., Jr, Fish M. B., Pollycove M., O'Day D. N. Ocular blood flow in experimentally induced immunogenic uveitis. Arch Ophthalmol. 1974 Jan;91(1):60–65. doi: 10.1001/archopht.1974.03900060064016. [DOI] [PubMed] [Google Scholar]
- Basu P. K., Minta J. O. Chemotactic migration of leucocytes through corneal layers: an in vitro study. Can J Ophthalmol. 1976 Jul;11(3):235–240. [PubMed] [Google Scholar]
- Becker E. L., Henson P. M. In vitro studies of immunologically induced secretion of mediators from cells and related phenomena. Adv Immunol. 1973;17:93–193. doi: 10.1016/s0065-2776(08)60732-4. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A., Rodrigues M. M., Gery I., Schiffmann E. Induction of ocular inflammation by synthetic mediators. Arch Ophthalmol. 1981 Aug;99(8):1436–1444. doi: 10.1001/archopht.1981.03930020310024. [DOI] [PubMed] [Google Scholar]
- Bhattacherjee P., Hammond B., Salmon J. A., Stepney R., Eakins K. E. Chemotactic response to some arachidonic acid lipoxygenase products in the rabbit eye. Eur J Pharmacol. 1981 Jul 17;73(1):21–28. doi: 10.1016/0014-2999(81)90141-2. [DOI] [PubMed] [Google Scholar]
- Brozna J. P., Senior R. M., Kreutzer D. L., Ward P. A. Chemotactic factor inactivators of human granulocytes. J Clin Invest. 1977 Dec;60(6):1280–1288. doi: 10.1172/JCI108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J. W., Heise E. R., Weiser R. S. Induction of delayed-type sensitivity-like reactions in the eye by the injection of lymphokines. Invest Ophthalmol. 1973 Jun;12(6):400–409. [PubMed] [Google Scholar]
- Desai U., Kreutzer D. L., Showell H., Arroyave C. V., Ward P. A. Acute inflammatory pulmonary reactions induced by chemotactic factors. Am J Pathol. 1979 Jul;96(1):71–83. [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Gamble C. N., Aronson B., Brescia F. S. Experimental uveitis. II. The pathogenesis of recurrent immunologic (Auer) uveitis. Arch Ophthalmol. 1970 Sep;84(3):331–341. doi: 10.1001/archopht.1970.00990040333013. [DOI] [PubMed] [Google Scholar]
- Grant J. A., Settle L., Whorton E. B., Dupree E. Complement-mediated release of histamine from human basophils. II. Biochemical characterization of the reaction. J Immunol. 1976 Aug;117(2):450–456. [PubMed] [Google Scholar]
- Howell S. B., Epstein W. V. Circulating immunoglobulin complexes in Wegener's granulomatosis. Am J Med. 1976 Feb;60(2):259–268. doi: 10.1016/0002-9343(76)90435-6. [DOI] [PubMed] [Google Scholar]
- Howes E. L., Jr, McKay D. G. Circulating immune complexes. Effects on ocular vascular permeability in the rabbit. Arch Ophthalmol. 1975 May;93(5):365–370. doi: 10.1001/archopht.1975.01010020377013. [DOI] [PubMed] [Google Scholar]
- Howes E. L., Jr, McKay D. G. Ocular changes in the generalized Shwartzman reaction. Arch Ophthalmol. 1973 Sep;90(3):218–224. doi: 10.1001/archopht.1973.01000050220007. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Anderson T. P., Ward P. A. Suppression of immune complex-induced inflammation by the chemotactic factor inactivator. J Clin Invest. 1977 May;59(5):951–958. doi: 10.1172/JCI108717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974 Aug;54(2):349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., O'Flaherty J. T., Orr W., Showell H. J., Ward P. A., Becker E. L. Quantitative comparisons of various biological responses of neutrophils to different active and inactive chemotactic factors. Immunopharmacology. 1978 Dec;1(1):39–47. doi: 10.1016/0162-3109(78)90007-3. [DOI] [PubMed] [Google Scholar]
- Levine R. A., Ward P. A. Experimental acute immunologic ocular vasculitis. Am J Ophthalmol. 1970 Jun;69(6):1023–1031. doi: 10.1016/0002-9394(70)91052-4. [DOI] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondino B. J., Ratajczak H. V., Goldberg D. B., Schanzlin D. J., Brown S. I. Alternate and classical pathway components of complement in the normal cornea. Arch Ophthalmol. 1980 Feb;98(2):346–349. doi: 10.1001/archopht.1980.01020030342023. [DOI] [PubMed] [Google Scholar]
- Reid T., Kenney C., Waring G. O. Isolation and characterization of fibronectin from bovine aqueous humor. Invest Ophthalmol Vis Sci. 1982 Jan;22(1):57–61. [PubMed] [Google Scholar]
- SILVERSTEIN A. M., ZIMMERMAN L. E. Immunogenic endophthalmitis produced in the guinea pig by different pathogenetic mechanisms. Am J Ophthalmol. 1959 Nov;48(5):435–447. doi: 10.1016/0002-9394(59)90596-3. [DOI] [PubMed] [Google Scholar]
- SZULMAN A. E. The histological distribution of blood group substances A and B in man. J Exp Med. 1960 Jun 1;111:785–800. doi: 10.1084/jem.111.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer H., Ward P. A. Lung and dermal vascular injury produced by preformed immune complexes. Am Rev Respir Dis. 1978 Mar;117(3):551–557. doi: 10.1164/arrd.1978.117.3.551. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- WAKSMAN B. H., BULLINGTON S. J. A quantitative study of the passive Arthus reaction in the rabbit eye. J Immunol. 1956 Jun;76(6):441–453. [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G. BOUND COMPLEMENT AND IMMUNOLOGIC INJURY OF BLOOD VESSELS. J Exp Med. 1965 Feb 1;121:215–234. doi: 10.1084/jem.121.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. Biologic role of complement products. Complement-derived leukotactic activity extractable from lesions of immunologic vasculitis. J Immunol. 1972 May;108(5):1137–1145. [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Williams B. D., Lehner T. Immune complexes in Behçet's syndrome and recurrent oral ulceration. Br Med J. 1977 May 28;1(6073):1387–1389. doi: 10.1136/bmj.1.6073.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Nydegger U., Perrin L. H., Fehr K., McCormick J., Lambert P. H., Miescher P. A. Circulating and intra-articular immune complexes in patients with rheumatoid arthritis. Correlation of 125I-Clq binding activity with clinical and biological features of the disease. J Clin Invest. 1976 May;57(5):1308–1319. doi: 10.1172/JCI108399. [DOI] [PMC free article] [PubMed] [Google Scholar]