Abstract
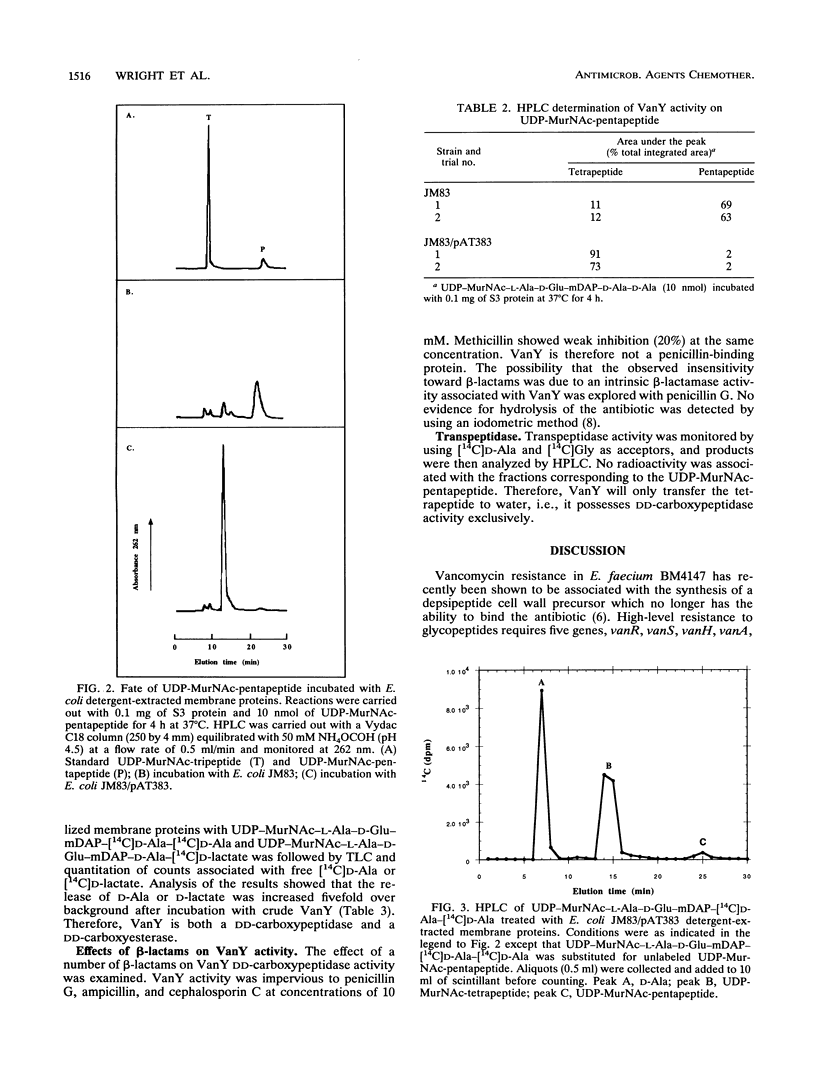
VanY is a protein with a molecular mass of 34.8 kDa encoded by vanY, a member of the high-level vancomycin resistance gene cluster found on plasmid pIP816 in Enterococcus faecium BM4147. Extracts from Escherichia coli JM83 bearing plasmid pAT383, which contains the vanY gene, were examined for enzymatic hydrolysis of peptidoglycan precursors. VanY was associated with the cell membranes and cleaved the C-terminal D-alanine residue of UDP-muramyl-pentapeptide but did not display transpeptidase or beta-lactamase activities. The DD-carboxypeptidase activity was not inhibited by beta-lactam antibiotics. VanY released the C-terminal D-hydroxy acid from depsipeptides produced by the vancomycin resistance protein VanA. These results demonstrate that VanY should contribute in vivo to the hydrolysis of both the D-alanyl-D-alanine- and the depsipeptide-containing peptidoglycan precursors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M., Molinas C., Bugg T. D., Wright G. D., Walsh C. T., Courvalin P. Evidence for in vivo incorporation of D-lactate into peptidoglycan precursors of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992 Apr;36(4):867–869. doi: 10.1128/aac.36.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Molinas C., Courvalin P. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1992 Apr;174(8):2582–2591. doi: 10.1128/jb.174.8.2582-2591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg T. D., Dutka-Malen S., Arthur M., Courvalin P., Walsh C. T. Identification of vancomycin resistance protein VanA as a D-alanine:D-alanine ligase of altered substrate specificity. Biochemistry. 1991 Feb 26;30(8):2017–2021. doi: 10.1021/bi00222a002. [DOI] [PubMed] [Google Scholar]
- Bugg T. D., Wright G. D., Dutka-Malen S., Arthur M., Courvalin P., Walsh C. T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 1991 Oct 29;30(43):10408–10415. doi: 10.1021/bi00107a007. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Frére J. M., Leyh-Bouille M., Ghuysen J. M., Nieto M., Perkins H. R. Exocellular DD-carboxypeptidases-transpeptidases from Streptomyces. Methods Enzymol. 1976;45:610–636. doi: 10.1016/s0076-6879(76)45054-1. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y., Ryono D. E., Neubeck R., Gordon E. M., Pluscec J. Streptomyces R61 DD-carboxypeptidase: hydrolysis of X-D-alanyl-D-alanine peptides measured by a fluorometric assay. Anal Biochem. 1984 Feb;137(1):125–128. doi: 10.1016/0003-2697(84)90357-9. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Billot-Klein D., al-Obeid S., Klare I., Francoual S., Collatz E., van Heijenoort J. Inducible carboxypeptidase activity in vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992 Jan;36(1):77–80. doi: 10.1128/aac.36.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Bingen E., Su Q. H., Lambert-Zechovski N., Courvalin P., Duval J. Effects of combinations of beta-lactams, daptomycin, gentamicin, and glycopeptides against glycopeptide-resistant enterococci. Antimicrob Agents Chemother. 1991 Jan;35(1):92–98. doi: 10.1128/aac.35.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr The Garrod Lecture. The enterococcus: a classic example of the impact of antimicrobial resistance on therapeutic options. J Antimicrob Chemother. 1991 Jul;28(1):1–12. doi: 10.1093/jac/28.1.1. [DOI] [PubMed] [Google Scholar]
- Popieniek P. H., Pratt R. F. A fluorescent ligand for binding studies with glycopeptide antibiotics of the vancomycin class. Anal Biochem. 1987 Aug 15;165(1):108–113. doi: 10.1016/0003-2697(87)90207-7. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1989 Nov;8(11):943–950. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Etter L., Gutmann L. Synergistic killing of vancomycin-resistant enterococci of classes A, B, and C by combinations of vancomycin, penicillin, and gentamicin. Antimicrob Agents Chemother. 1991 Apr;35(4):776–779. doi: 10.1128/aac.35.4.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Al-Obeid S., Shlaes J. H., Goldstein F. W., Shlaes D. M. Inducible resistance to vancomycin in Enterococcus faecium D366. J Infect Dis. 1989 Jun;159(6):1095–1104. doi: 10.1093/infdis/159.6.1095. [DOI] [PubMed] [Google Scholar]
- al-Obeid S., Collatz E., Gutmann L. Mechanism of resistance to vancomycin in Enterococcus faecium D366 and Enterococcus faecalis A256. Antimicrob Agents Chemother. 1990 Feb;34(2):252–256. doi: 10.1128/aac.34.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]