Abstract
Cellular suspensions (2 x 10(6) cells) of isolated preneoplastic liver cells, obtained from carcinogen-treated rats, were injected in the spleens of syngeneic rats divided into groups on the basis of no treatment, partial hepatectomy (PH), and/or feeding regimens including 2-acetylaminofluorene (AAF). Recipient rats undergoing both PH and AAF showed significantly more rapid proliferation of the preneoplastic liver cell implant, compared with other treatment groups and control. The theoretic basis for this observation, supported by a large body of data derived from hepatocarcinogenesis, is as follows: The phenotype of the donor cells has been altered by chemical carcinogens such that the liver cells develop resistance to growth-inhibiting agents such as AAF. The recipient receives PH and AAF, the former creating a strong proliferative stimulus for hepatocytes, while the latter inhibits regeneration of normal liver cells but not those resistant to the mito-inhibitory effect of AAF, ie, the carcinogen-altered donor cells. These manipulations in donors and recipients thus create a selective environment in which the implant undergoes rapid proliferation. This model of resistance induction followed by selective proliferation, built upon the principles of carcinogenesis and applied to isolated liver cell transplantation, provides an experimental basis for achieving rapid liver growth of the splenic implant.
Full text
PDF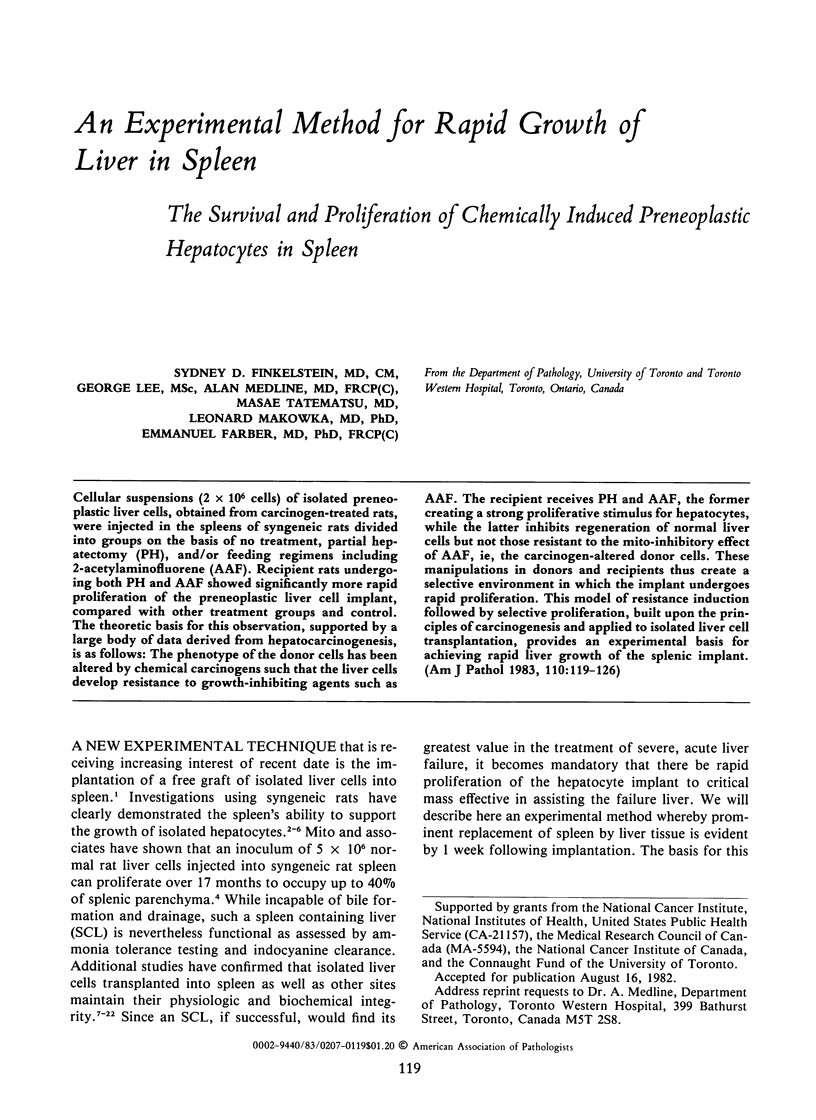





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebata H., Kusano M., Onishi T., Saito T., Mito M. Liver regeneration utilizing isolated hepatocytes transplanted into the rat spleen. Surg Forum. 1978;29:338–340. [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Farber E. Chemical carcinogenesis: a biologic perspective. Am J Pathol. 1982 Feb;106(2):271–296. [PMC free article] [PubMed] [Google Scholar]
- Farber E., Parker S., Gruenstein M. The resistance of putative premalignant liver cell populations, hyperplastic nodules, to the acute cytotoxic effects of some hepatocarcinogens. Cancer Res. 1976 Nov;36(11 Pt 1):3879–3887. [PubMed] [Google Scholar]
- Groth C. G., Arborgh B., Björkén C., Sundberg B., Lundgren G. Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rat by intraportal hepatocyte transplantation. Transplant Proc. 1977 Mar;9(1):313–316. [PubMed] [Google Scholar]
- Kusano M., Mito M. Observations on the fine structure of long-survived isolated hepatocytes inoculated into rat spleen. Gastroenterology. 1982 Apr;82(4):616–628. [PubMed] [Google Scholar]
- Laishes B. A., Williams G. M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. 1. The effect of insulin. In Vitro. 1976 Jul;12(7):521–532. doi: 10.1007/BF02796495. [DOI] [PubMed] [Google Scholar]
- Löwenberg B., Vossen J. M., Dooren L. J. Transplantation of fetal liver cells in the treatment of severe combined immunodeficiency disease. Blut. 1977 Mar;34(3):181–195. doi: 10.1007/BF00996167. [DOI] [PubMed] [Google Scholar]
- Makowka L., Rotstein L. E., Falk R. E., Falk J. A., Langer B., Nossal N. A., Blendis L. M., Phillips M. J. Reversal of toxic and anoxic induced hepatic failure by syngeneic, allogeneic, and xenogeneic hepatocyte transplantation. Surgery. 1980 Aug;88(2):244–253. [PubMed] [Google Scholar]
- Matas A. J., Sutherland D. E., Steffes M. W., Mauer S. M., Sowe A., Simmons R. L., Najarian J. S. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976 May 28;192(4242):892–894. doi: 10.1126/science.818706. [DOI] [PubMed] [Google Scholar]
- Matas A. J., Sutherland D. E., Steffes M. W., Simmons R. L., Najarian J. S. Hepatocellular transplantation in UDP-glucuronyl-transferase-deficient rats. Surg Forum. 1975;26:428–431. [PubMed] [Google Scholar]
- Mito M., Ebata H., Kusano M., Onishi T., Hiratsuka M., Saito T. Studies on ectopic liver utilizing hepatocyte transplantation into the rat spleen. Transplant Proc. 1979 Mar;11(1):585–591. [PubMed] [Google Scholar]
- Mito M., Ebata H., Kusano M., Onishi T., Saito T., Sakamoto S. Morphology and function of isolated hepatocytes transplanted into rat spleen. Transplantation. 1979 Dec;28(6):499–505. doi: 10.1097/00007890-197912000-00013. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Krasner J. Induction of an enzyme in genetically deficient rats after grafting of normal liver. Science. 1973 Oct 5;182(4107):68–70. doi: 10.1126/science.182.4107.68. [DOI] [PubMed] [Google Scholar]
- Numata M., Sutherland D. E., Matas A. J., Simmons R. L., Najarian J. S. Allogeneic liver cell transplantation for experimental acute hepatic failure. Surg Forum. 1977;28:307–309. [PubMed] [Google Scholar]
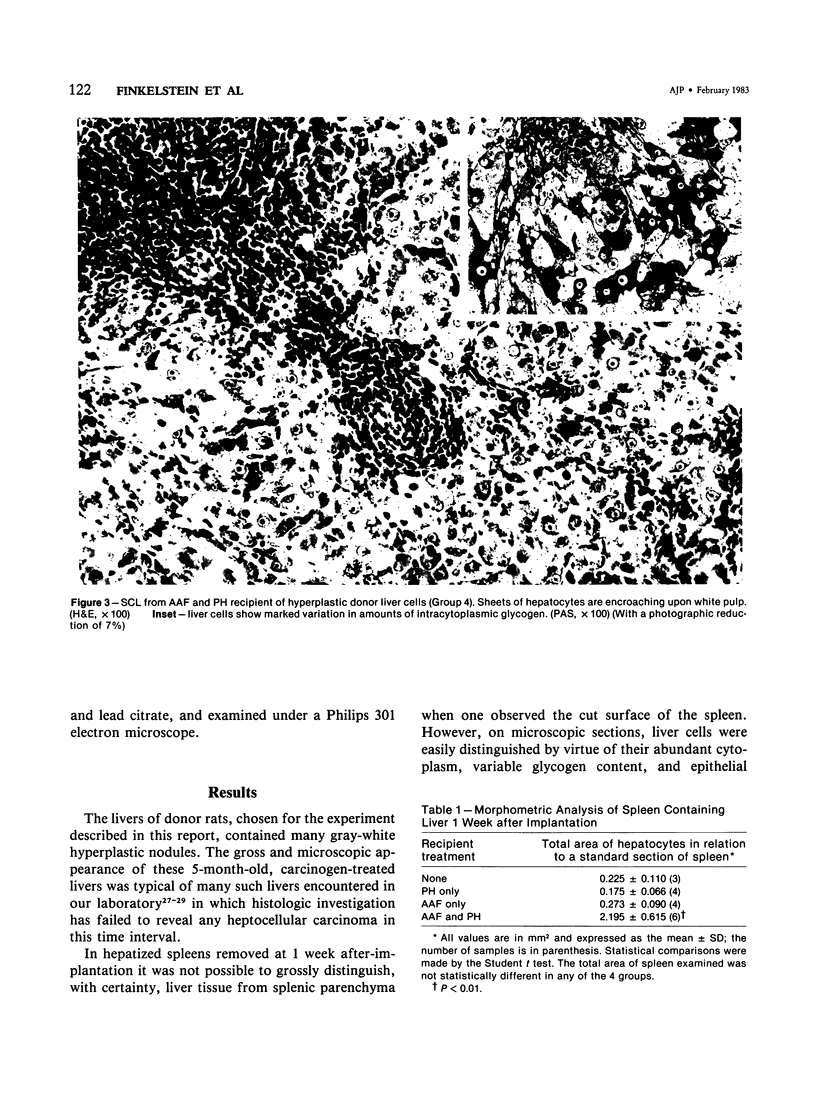
- Ogawa K., Medline A., Farber E. Sequential analysis of hepatic carcinogenesis: a comparative study of the ultrastructure of preneoplastic, malignant, prenatal, postnatal, and regenerating liver. Lab Invest. 1979 Jul;41(1):22–35. [PubMed] [Google Scholar]
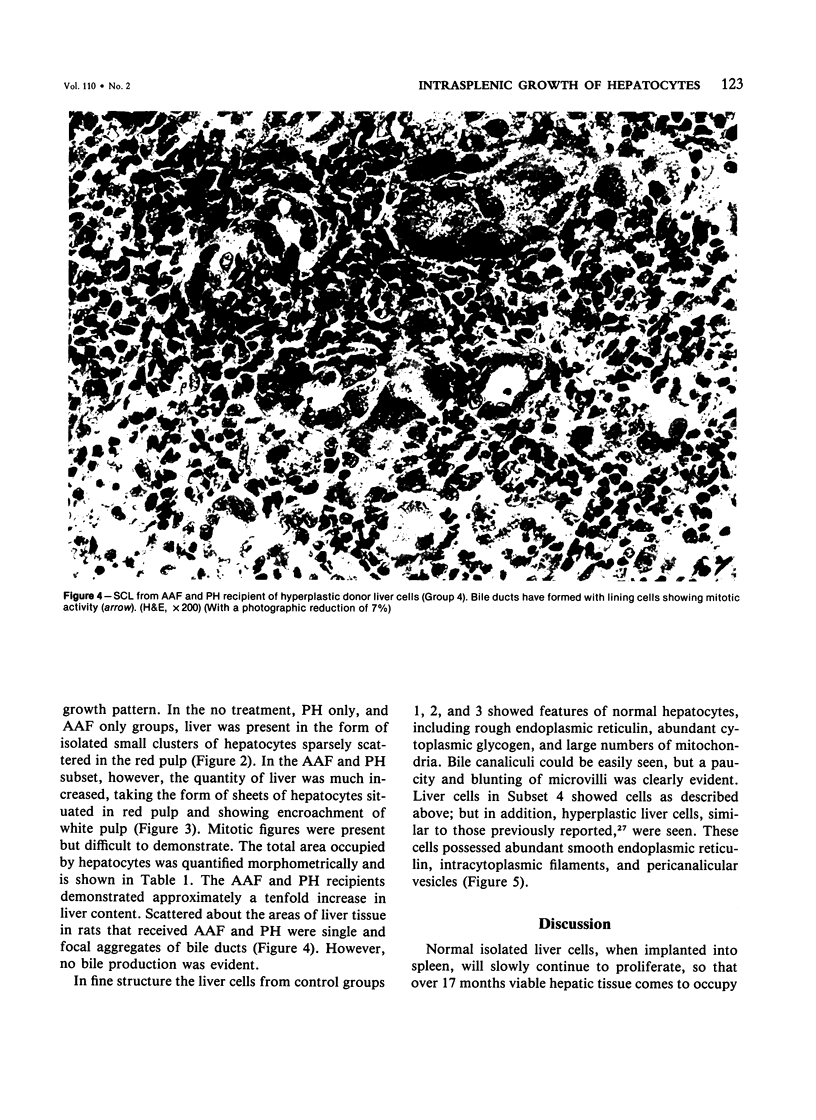
- Ogawa K., Solt D. B., Farber E. Phenotypic diversity as an early property of putative preneoplastic hepatocyte populations in liver carcinogenesis. Cancer Res. 1980 Mar;40(3):725–733. [PubMed] [Google Scholar]
- Rugstad H. E., Robinson S. H., Yannoni C., Tashjian A. H., Jr Transfer of bilirubin uridine diphosphate-glucuronyltransferase to enzyme-deficient rats. Science. 1970 Oct 30;170(3957):553–555. doi: 10.1126/science.170.3957.553. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Sommer B. G., Sutherland D. E., Matas A. J., Simmons R. L., Najarian J. S. Hepatocellular transplantation for treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc. 1979 Mar;11(1):578–584. [PubMed] [Google Scholar]
- Sommer B. G., Sutherland D. E., Simmons R. L., Najarian J. S. Hepatocellular transplantation for experimental acute liver failure in dogs. Surg Forum. 1979;30:279–281. [PubMed] [Google Scholar]
- Sommer B. G., Sutherland D. E., Simmons R. L., Najarian J. S. Hepatocellular transplantation for experimental ischemic acute liver failure in dogs. J Surg Res. 1980 Oct;29(4):319–325. doi: 10.1016/0022-4804(80)90064-5. [DOI] [PubMed] [Google Scholar]
- Sutherland D. E., Matas A. J., Najarian J. S. The mutual impact of transplantation and advances in the understanding and treatment of metabolic diseases. Transplant Proc. 1980 Dec;12(4):643–652. [PubMed] [Google Scholar]
- Sutherland D. E., Matas A. J., Steffes M. W., Simmons R. L., Najarian J. S. Transplantation of liver cells in an animal model of congenital enzyme deficiency disease: the Gunn rat. Transplant Proc. 1977 Mar;9(1):317–319. [PubMed] [Google Scholar]
- Sutherland D. E., Numata M., Matas A. J., Simmons R. L., Najarian J. S. Hepatocellular transplantation in acute liver failure. Surgery. 1977 Jul;82(1):124–132. [PubMed] [Google Scholar]
- Williams G. M., Klaiber M., Farber E. Differences in growth of transplants of liver, liver hyperplastic nodules, and hepatocellular carcinomas in the mammary fat pad. Am J Pathol. 1977 Nov;89(2):379–390. [PMC free article] [PubMed] [Google Scholar]
- Williams G. M., Ohmori T., Watanabe K. The persistence and phenotypic stability of transplanted rat liver neoplastic nodules. Am J Pathol. 1980 Apr;99(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- Woods R. J., Fuller B. J., Attenburrow V. D., Nutt L. H., Hobbs K. E. Functional assessment of hepatocytes after transplantation into rat spleen.. Transplantation. 1982 Feb;33(2):123–126. doi: 10.1097/00007890-198202000-00004. [DOI] [PubMed] [Google Scholar]