Abstract
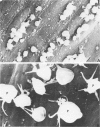
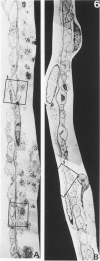
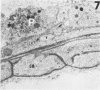
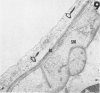
Those microvascular endothelial events that parallel the evolution of platelet aggregation were evaluated in a well-controlled animal model. Cat pial microvessels were observed through a cranial window while local platelet aggregation was produced by intravenous injection of sodium fluorescein and simultaneous exposure of the pial vessels to light from a filtered mercury lamp that excited the fluorescein. The vessels were fixed in situ when the in vivo observations of a preselected vessel indicated early, intermediate, or advanced aggregation in that vessel. The preselected vessel was then harvested for ultrastructural study together with adjacent vessels from the illuminated field. These vessels and appropriate controls were compared in semiserial thin sections. The onset of platelet aggregation in both venules and arterioles was accompanied by focal endothelial lucency, vacuole formation, luminal membrane rupture, and swelling of the nuclear envelope. These changes were not found in control material. With intermediate aggregation these changes were more common, while with advanced aggregation these abnormalities occurred together with focal endothelial denudation. Thus, in this model denudation occurred only with advanced aggregation and was not a prerequisite for aggregation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner H. R., Turitto V., Weiss H. J. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. II. Relationships among platelet adhesion, thrombus dimensions, and fibrin formation. J Lab Clin Med. 1980 Feb;95(2):208–221. [PubMed] [Google Scholar]
- Honour A. J., Pickering G. W., Sheppard B. L. Ultrastructure and behaviour of platelet thrombi in injured arteries. Br J Exp Pathol. 1971 Oct;52(5):482–494. [PMC free article] [PubMed] [Google Scholar]
- Hornstra G. Platelet - vessel wall interaction: role of blood clotting. Philos Trans R Soc Lond B Biol Sci. 1981 Aug 18;294(1072):355–371. doi: 10.1098/rstb.1981.0112. [DOI] [PubMed] [Google Scholar]
- Hovig T., McKenzie F. N., Arfors K. E. Measruement of the platelet response to laserinduced microvascular injury. Ultrastructural studies. Thromb Diath Haemorrh. 1974 Dec 31;32(2-3):695–703. [PubMed] [Google Scholar]
- Huang T. W., Benditt E. P. Mechanisms of platelet adhesion to the basal lamina. Am J Pathol. 1978 Jul;92(1):99–110. [PMC free article] [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Povlishock J. T., Dietrich W. D., Magiera C. J., Ellis E. F. Cerebral arteriolar damage by arachidonic acid and prostaglandin G2. Science. 1980 Sep 12;209(4462):1242–1245. doi: 10.1126/science.7403881. [DOI] [PubMed] [Google Scholar]
- Meyers K. M., Hopkins G., Holmsen H., Benson K., Prieur D. J. Ultrastructure of resting and activated storage pool deficient platelets from animals with the Chédiak-Higashi syndrome. Am J Pathol. 1982 Mar;106(3):364–377. [PMC free article] [PubMed] [Google Scholar]
- PEASE D. C., MOLINARI S. Electron microscopy of muscular arteries; pial vessels of43 the cat and monkey. J Ultrastruct Res. 1960 Jun;3:447–468. doi: 10.1016/s0022-5320(60)90022-8. [DOI] [PubMed] [Google Scholar]
- Ratliff N. B., Gerrard J. M., White J. G. Platelet-leukocyte interactions following arterial endothelial injury. Am J Pathol. 1979 Aug;96(2):567–580. [PMC free article] [PubMed] [Google Scholar]
- Rosenblum W. I., El-Sabban F. Dimethyl sulfoxide (DMSO) and glycerol, hydroxyl radical scavengers, impair platelet aggregation within and eliminate the accompanying vasodilation of, injured mouse pial arterioles. Stroke. 1982 Jan-Feb;13(1):35–39. doi: 10.1161/01.str.13.1.35. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I., El-Sabban F., Ellis E. F. Aspirin and indomethacin, nonsteroidal antiinflammatory agents alter the responses to microvascular injury in brain and mesentery. Microvasc Res. 1980 Nov;20(3):374–378. doi: 10.1016/0026-2862(80)90066-7. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I., El-Sabban F. Enhancement of platelet aggregation by tranylcypromine in mouse cerebral microvessels. Circ Res. 1978 Aug;43(2):238–241. doi: 10.1161/01.res.43.2.238. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I., El-Sabban F. Platelet aggregation in the cerebral microcirculation: effect of aspirin and other agents. Circ Res. 1977 Mar;40(3):320–328. doi: 10.1161/01.res.40.3.320. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I., El-Sabban F. Topical prostacyclin (PGI2) inhibits platelet aggregation in pial venules of the mouse. Stroke. 1979 Jul-Aug;10(4):399–401. doi: 10.1161/01.str.10.4.399. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I., El-Sabban F. Use of AHR-5850 and AHR-6293 to distinguish the effect of anti-platelet aggregating drug properties from the effect of anti-inflammatory properties on an in vivo model of platelet aggregation. Microvasc Res. 1979 May;17(3 Pt 1):309–313. doi: 10.1016/s0026-2862(79)80006-0. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I. Fluorescence induced in platelet aggregates as a guide to luminal contours in the presence of platelet aggregation. Microvasc Res. 1978 Jan;15(1):103–106. doi: 10.1016/0026-2862(78)90010-9. [DOI] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Stemerman M. B., Benditt E. P. The aortic intima. II. Repair of the aortic lining after mechanical denudation. Am J Pathol. 1975 Oct;81(1):15–42. [PMC free article] [PubMed] [Google Scholar]
- Still W. J., Dennison S. Arterial thrombosis induced by hypertension and fatty acid mobilization. Arch Pathol. 1972 Jul;94(1):23–28. [PubMed] [Google Scholar]
- Szalay J. Morphological response of blood platelets to increased venular permeability in vivo. Microvasc Res. 1981 Jan;21(1):57–74. doi: 10.1016/0026-2862(81)90005-4. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Carvalho A. C. Type IV and type "A-B" collagens do not elicit platelet aggregation or the serotonin release reaction. J Lab Clin Med. 1979 Mar;93(3):499–505. [PubMed] [Google Scholar]
- Wall R. T., Harker L. A. The endothelium and thrombosis. Annu Rev Med. 1980;31:361–371. doi: 10.1146/annurev.me.31.020180.002045. [DOI] [PubMed] [Google Scholar]
- Wei E. P., Dietrich W. D., Povlishock J. T., Navari R. M., Kontos H. A. Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ Res. 1980 Jan;46(1):37–47. doi: 10.1161/01.res.46.1.37. [DOI] [PubMed] [Google Scholar]
- Wei E. P., Kontos H. A., Dietrich W. D., Povlishock J. T., Ellis E. F. Inhibition by free radical scavengers and by cyclooxygenase inhibitors of pial arteriolar abnormalities from concussive brain injury in cats. Circ Res. 1981 Jan;48(1):95–103. doi: 10.1161/01.res.48.1.95. [DOI] [PubMed] [Google Scholar]
- Wiedeman M. P. Vascular reactions to laser in vivo. Microvasc Res. 1974 Sep;8(2):132–138. doi: 10.1016/0026-2862(74)90087-9. [DOI] [PubMed] [Google Scholar]