Abstract
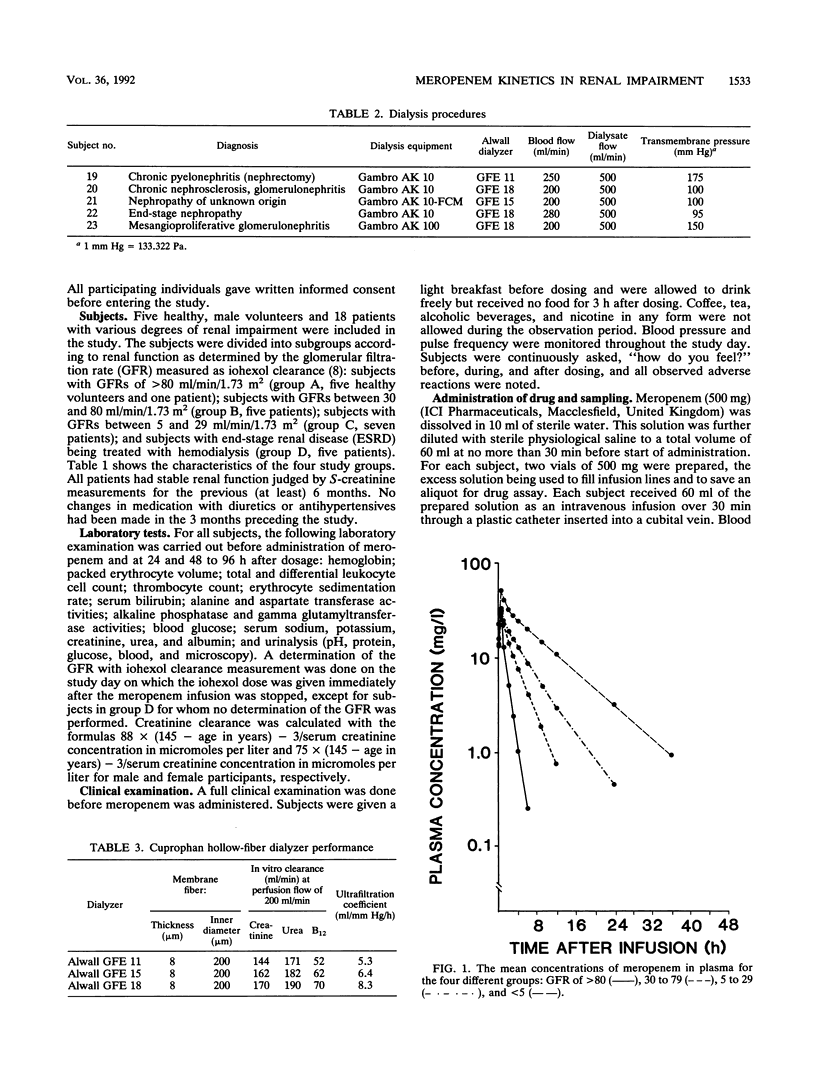
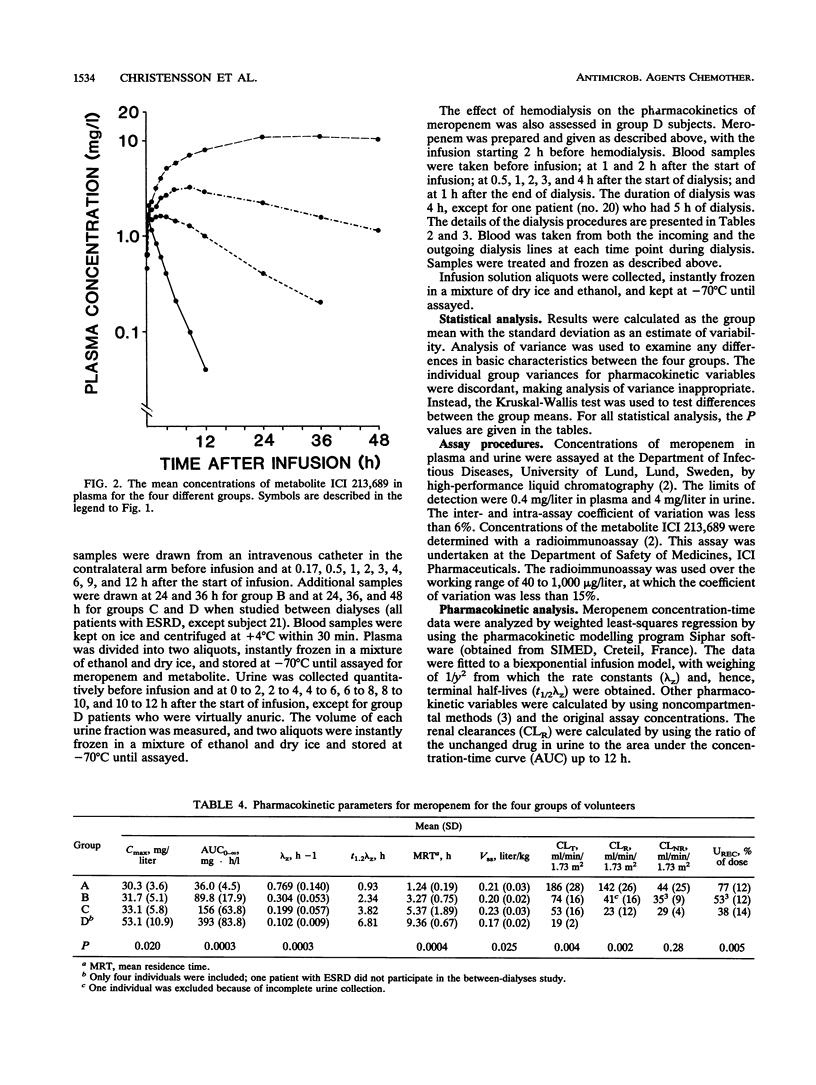
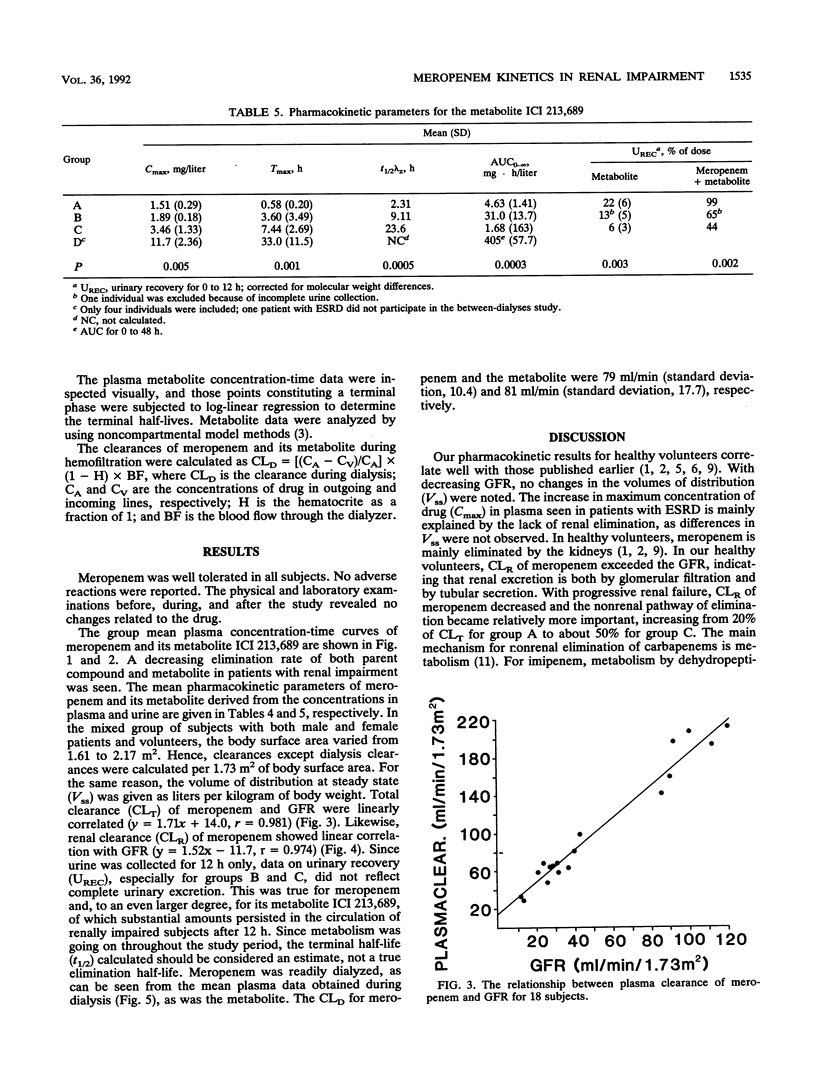
Five healthy volunteers and 18 patients with various degrees of renal impairment received 500 mg of meropenem intravenously as a 30-min infusion. Five dialysis patients were dosed 2 h prior to hemodialysis, and four of them were also dosed between hemodialysis treatments. Plasma and urine samples were collected for up to 48 h and 12 h, respectively. Concentrations of meropenem and its open ring metabolite ICI 213,689 were determined by high-performance liquid chromatography and radioimmunoassay, respectively. The subjects were divided into four groups with glomerular filtration rates (GFR) of greater than 80, 30 to 80, 5 to 29, or less than 5 ml/min. There were linear correlations between the GFR and the rates for total plasma clearance as well as renal clearance of meropenem (group mean values for total clearance of 186, 74, 53, and 19 ml/min/1.73 m2, respectively). In subjects with normal renal function, nonrenal clearance accounted for approximately 20% of total elimination, increasing to about 50% in patients with GFR between 5 and 29 ml/min/1.73 m2. The terminal half-life of meropenem increased from 0.9 h in the healthy volunteers to 6.8 h in patients with end-stage renal disease. The half-life of ICI 213,689 was 2.31 h in the healthy volunteers and increased to 23.6 h in patients with GFR of 5 to 29 ml/min. In patients with end-stage renal disease, half-lives could not be measured, as concentrations were hardly declining during the 48-h observation period. The area under the concentration-time curve for meropenem increased more than 10-fold. Both meropenem and its open ring metabolite were readily dialyzable, with dialysis clearances of 79 and 81 ml/min/1.73 m2, respectively.
Full text
PDF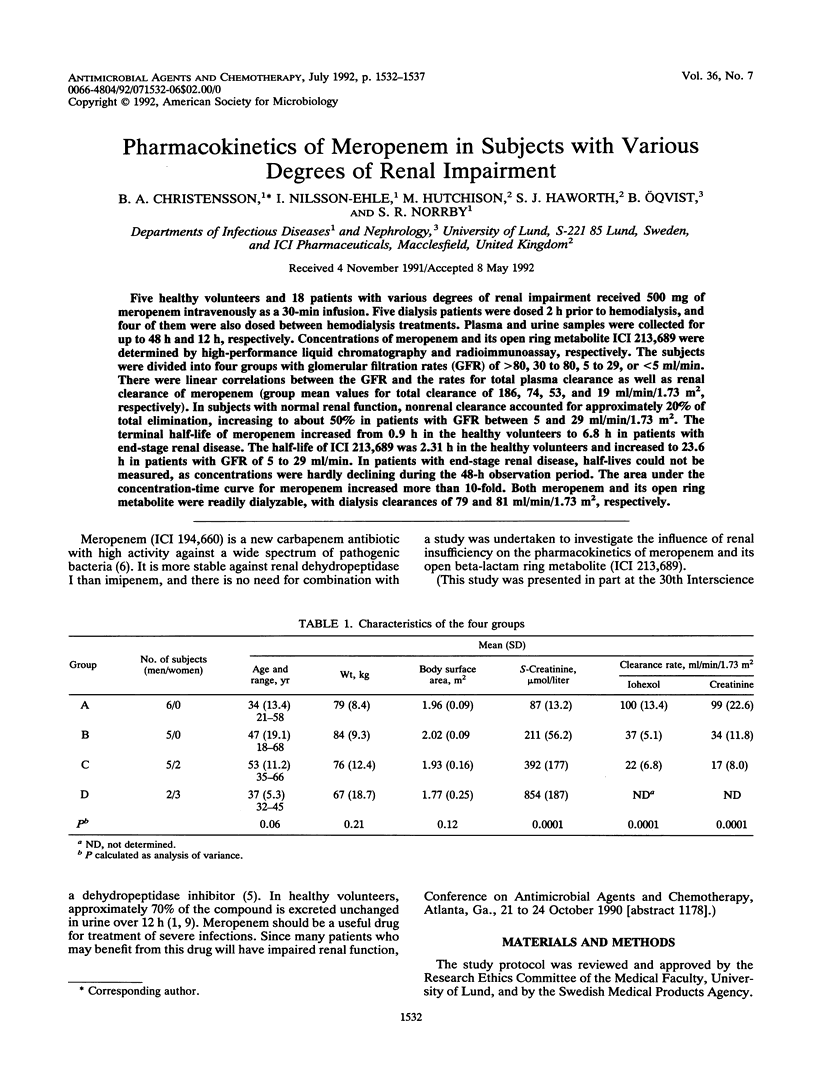

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax R. P., Bastain W., Featherstone A., Wilkinson D. M., Hutchison M., Haworth S. J. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):311–320. doi: 10.1093/jac/24.suppl_a.311. [DOI] [PubMed] [Google Scholar]
- Burman L. A., Nilsson-Ehle I., Hutchison M., Haworth S. J., Norrby S. R. Pharmacokinetics of meropenem and its metabolite ICI 213,689 in healthy subjects with known renal metabolism of imipenem. J Antimicrob Chemother. 1991 Feb;27(2):219–224. doi: 10.1093/jac/27.2.219. [DOI] [PubMed] [Google Scholar]
- Gibson T. P., Demetriades J. L., Bland J. A. Imipenem/cilastatin: pharmacokinetic profile in renal insufficiency. Am J Med. 1985 Jun 7;78(6A):54–61. doi: 10.1016/0002-9343(85)90102-0. [DOI] [PubMed] [Google Scholar]
- Harrison M. P., Moss S. R., Featherstone A., Fowkes A. G., Sanders A. M., Case D. E. The disposition and metabolism of meropenem in laboratory animals and man. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):265–277. doi: 10.1093/jac/24.suppl_a.265. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C. In-vitro studies of meropenem. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):9–29. doi: 10.1093/jac/24.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- Kropp H., Sundelof J. G., Hajdu R., Kahan F. M. Metabolism of thienamycin and related carbapenem antibiotics by the renal dipeptidase, dehydropeptidase. Antimicrob Agents Chemother. 1982 Jul;22(1):62–70. doi: 10.1128/aac.22.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzén E., Bäck S. E., Nilsson-Ehle I., Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984 Dec;104(6):955–961. [PubMed] [Google Scholar]
- Nilsson-Ehle I., Hutchison M., Haworth S. J., Norrby S. R. Pharmacokinetics of meropenem compared to imipenem-cilastatin in young, healthy males. Eur J Clin Microbiol Infect Dis. 1991 Feb;10(2):85–88. doi: 10.1007/BF01964413. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Alestig K., Björnegård B., Burman L. A., Ferber F., Huber J. L., Jones K. H., Kahan F. M., Kahan J. S., Kropp H. Urinary recovery of N-formimidoyl thienamycin (MK0787) as affected by coadministration of N-formimidoyl thienamycin dehydropeptidase inhibitors. Antimicrob Agents Chemother. 1983 Feb;23(2):300–307. doi: 10.1128/aac.23.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Rogers J. D., Ferber F., Jones K. H., Zacchei A. G., Weidner L. L., Demetriades J. L., Gravallese D. A., Hsieh J. Y. Disposition of radiolabeled imipenem and cilastatin in normal human volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):707–714. doi: 10.1128/aac.26.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J. B., Giles R. E. Meropenem: evidence of lack of proconvulsive tendency in mice. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):307–309. doi: 10.1093/jac/24.suppl_a.307. [DOI] [PubMed] [Google Scholar]
- Schliamser S. E., Broholm K. A., Liljedahl A. L., Norrby S. R. Comparative neurotoxicity of benzylpenicillin, imipenem/cilastatin and FCE 22101, a new injectible penem. J Antimicrob Chemother. 1988 Nov;22(5):687–695. doi: 10.1093/jac/22.5.687. [DOI] [PubMed] [Google Scholar]
- Schliamser S. E., Cars O., Norrby S. R. Neurotoxicity of beta-lactam antibiotics: predisposing factors and pathogenesis. J Antimicrob Chemother. 1991 Apr;27(4):405–425. doi: 10.1093/jac/27.4.405. [DOI] [PubMed] [Google Scholar]
- Tse C. S., Hernandez Vera F., Desai D. V. Seizure-like activity associated with imipenem-cilastatin. Drug Intell Clin Pharm. 1987 Jul-Aug;21(7-8):659–660. doi: 10.1177/1060028087021007-820. [DOI] [PubMed] [Google Scholar]
- Williams P. D., Bennett D. B., Comereski C. R. Animal model for evaluating the convulsive liability of beta-lactam antibiotics. Antimicrob Agents Chemother. 1988 May;32(5):758–760. doi: 10.1128/aac.32.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]


