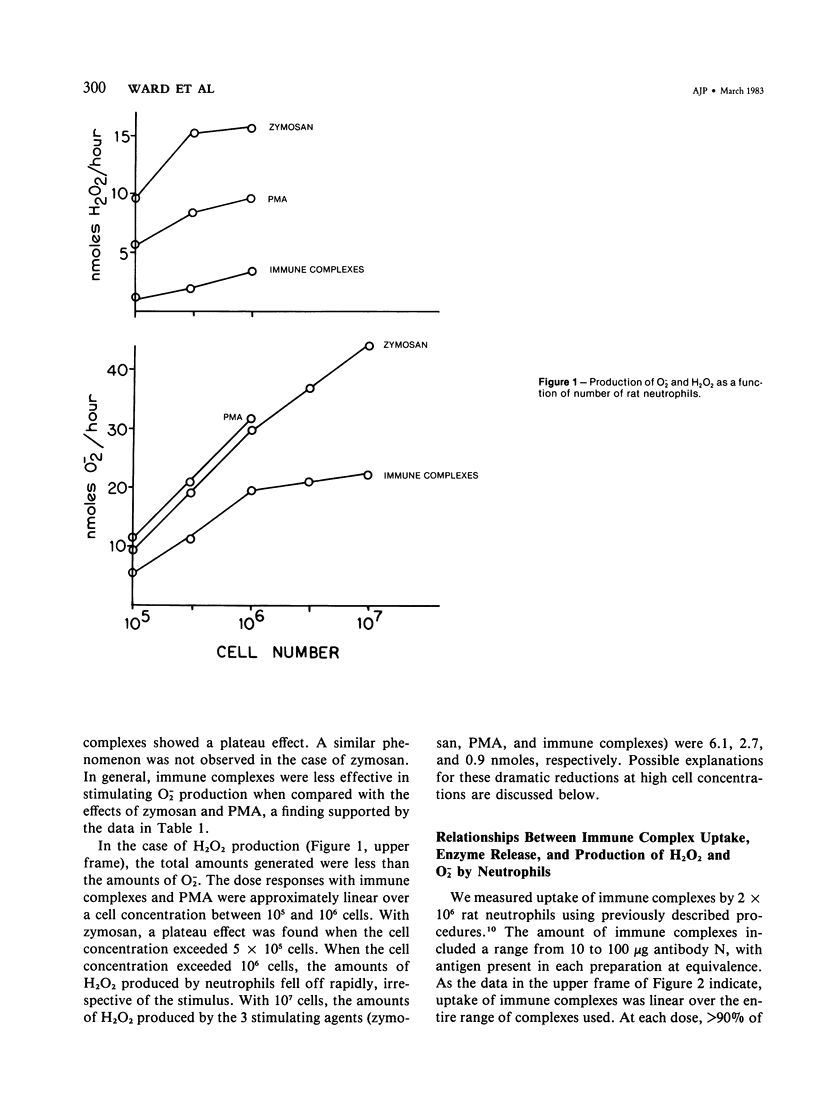
Abstract
Rat neutrophils and alveolar macrophages were quantitatively studied for production of O-2 and H2O2 after incubation of cells with immune complexes, and the responses were compared with those produced after cell contact with phorbal myristate acetate or zymosan particles. The production of toxic oxygen products is a linear function of cell number, the duration of incubation, and the amount of immune complex employed. In the case of neutrophils, there is a direct relationship between the amounts of immune complex internalized, secretory release of lysosomal enzymes, and production of O-2 and H2O2. With both neutrophils as well as alveolar macrophages, maximal production of O-2 occurs with the largest complexes (formed under conditions of antigen equivalence). When limiting cell concentrations are used, alveolar macrophages produce considerably more oxygen products than an equivalent number of peritoneal neutrophils obtained from the same animals. Thus, alveolar macrophages as well as neutrophils represent important potential sources for the generation of toxic oxygen products in lung inflammatory reactions. Experiments have also been designed to estimate the relative contributions of neutrophils and alveolar macrophages in vivo during acute immune complex deposition in lung. The data indicate that both neutrophils and alveolar macrophages are activated by in vivo exposure to immune complexes, each cell type producing a 2-4-fold increase (over baseline levels) in the amounts of O-2. Thus, alveolar macrophages as well as neutrophils may play an important role in the generation of toxic oxygen products that have been incriminated in the pathogenesis of acute lung injury following deposition of immune complexes.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCHRANE C. G., WEIGLE W. O., DIXON F. J. The role of polymorphonuclear leukocytes in the initiation and cessation of the Arthus vasculitis. J Exp Med. 1959 Sep 1;110:481–494. doi: 10.1084/jem.110.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute and progressive lung injury after contact with phorbol myristate acetate. Am J Pathol. 1982 Apr;107(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974 Aug;54(2):349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- McCormick J. R., Harkin M. M., Johnson K. J., Ward P. A. Suppression by superoxide dismutase of immune-complex--induced pulmonary alveolitis and dermal inflammation. Am J Pathol. 1981 Jan;102(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G. BOUND COMPLEMENT AND IMMUNOLOGIC INJURY OF BLOOD VESSELS. J Exp Med. 1965 Feb 1;121:215–234. doi: 10.1084/jem.121.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Quantitative phagocytosis by neutrophils. I. A new method with immune complexes. J Immunol. 1973 Dec;111(6):1771–1776. [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Quantitative phagocytosis by neutrophils. II. Release of the C5-cleaving enzyme and inhibition of phagocytosis by rheumatoid factor. J Immunol. 1973 Dec;111(6):1777–1782. [PubMed] [Google Scholar]
- Weiss S. J., Ward P. A. Immune complex induced generation of oxygen metabolites by human neutrophils. J Immunol. 1982 Jul;129(1):309–313. [PubMed] [Google Scholar]


