Abstract
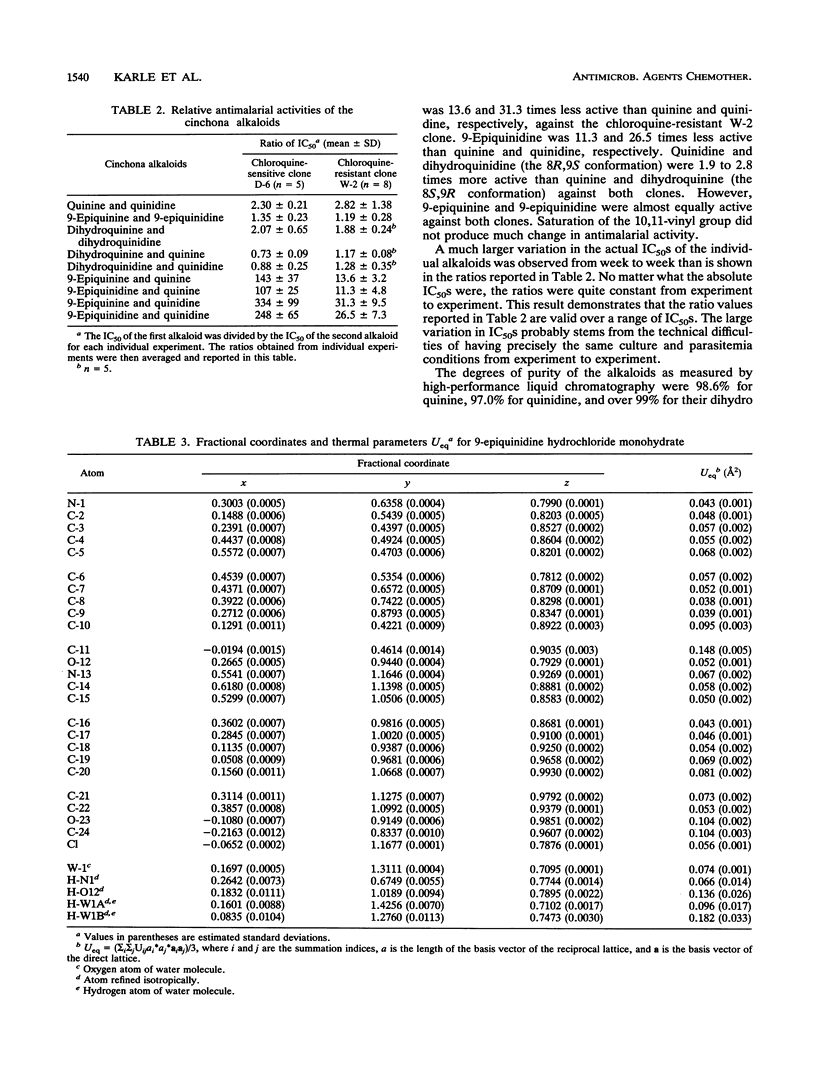
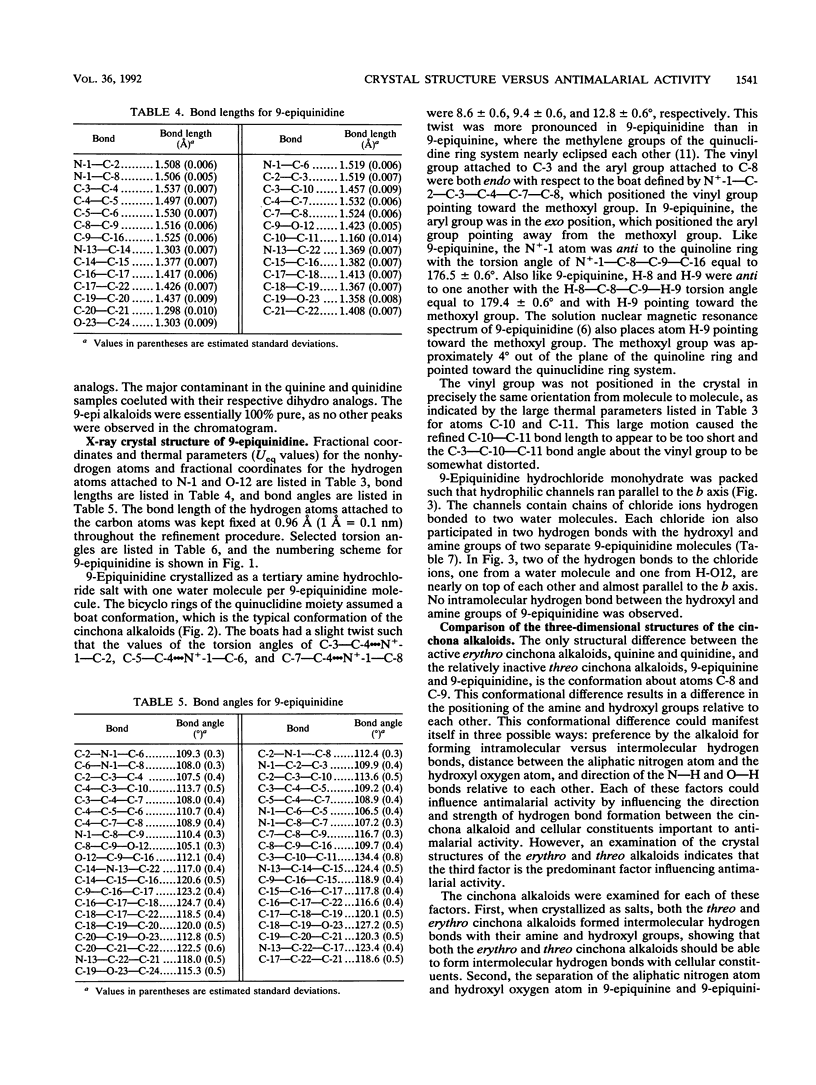
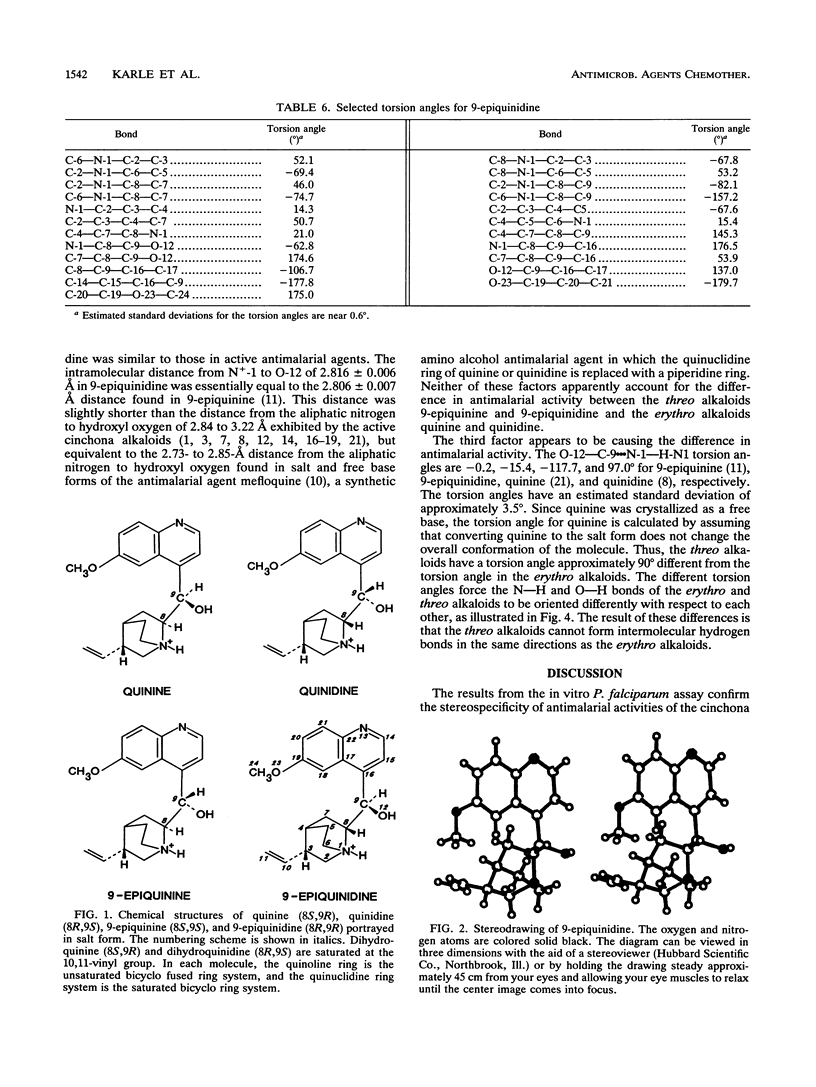
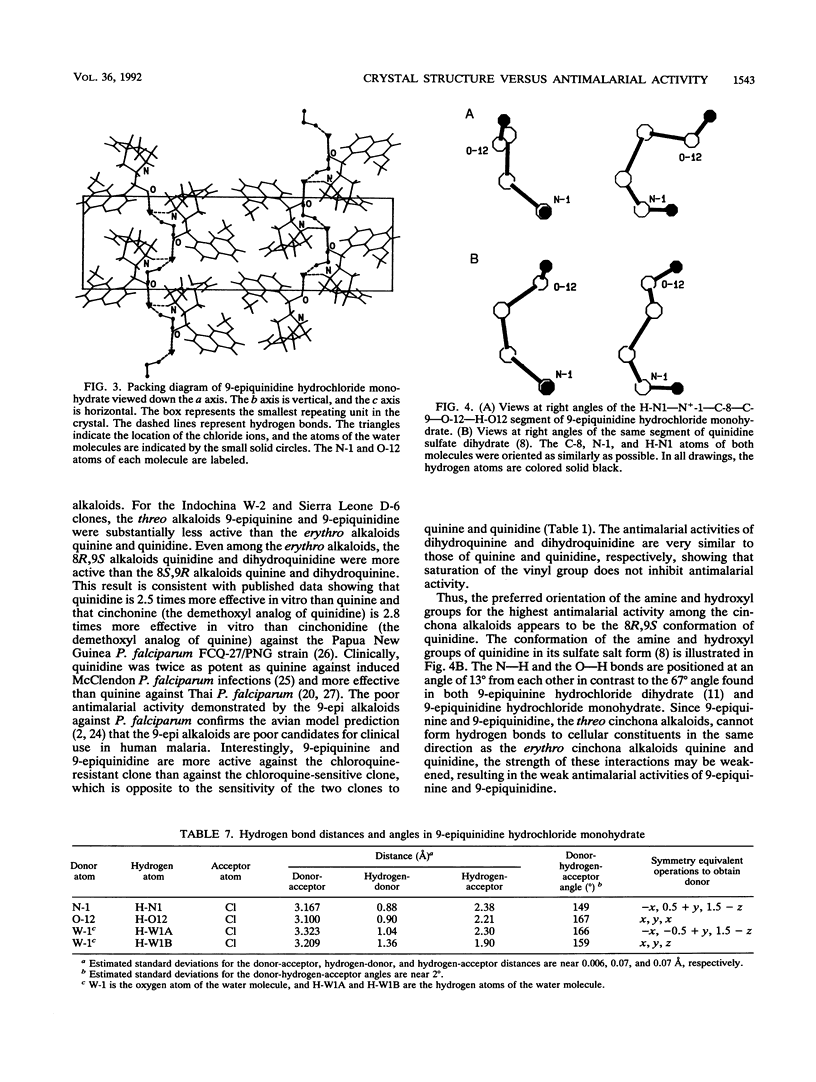
Quinine and quinidine were over 100 times more active than 9-epiquinine and 9-epiquinidine against chloroquine-sensitive Plasmodium falciparum and over 10 times more active against chloroquine-resistant P. falciparum. Since the only structural difference between quinine, quinidine, 9-epiquinine, and 9-epiquinidine is their three-dimensional configuration, the three-dimensional structures of these four alkaloids were examined in order to explain the large difference in relative activities between the 9-epi alkaloids and quinine and quinidine. The crystal structure of 9-epiquinidine hydrochloride monohydrate was determined by X-ray diffraction and was compared with the crystal structures of quinine, quinidine sulfate dihydrate, and 9-epiquinine hydrochloride dihydrate. The crystallographic parameters for 9-epiquinidine hydrochloride monohydrate were as follows: chemical formula, C20H25N2O2+.Cl-.H2O; M(r), 378.9; symmetry of unit cell, orthorhombic; space group, P2(1)2(1)2(1); parameters of unit cell, a was 7.042 +/- 0.001 A (1 A = 0.1 nm), b was 9.082 +/- 0.001 A, c was 31.007 +/- 0.005 A; the volume of unit cell was 1,983.1 +/- 0.6 A3; number of molecules per unit cell was 4; the calculated density was 1.27 g cm-3; the source of radiation was Cu K alpha (lambda = 1.54178 A); mu (absorption coefficient) was 18.82 cm-1; F(000) (sum of atomic scattering factors at zero scattering angle) was 808; room temperature was used; final R (residual index) was 5.72% for 1,501 reflections with magnitude of F(o) greater than 3 sigma (F). The intramolecular distance from N-1 to O-12 in 9-epiquinidine and 9-epiquinine, although shorter than the corresponding distance in quinine and quinidine, was similar to those of other active amino alcohol antimalarial agents. In all four alkaloids, both the hydroxyl and amine groups formed intermolecular hydrogen bonds, showing the potential for forming hydrogen bonds with cellular constituents. However, the positioning of the N+-1--H-N1 and O-12--H-O12 groups relative to each other was quite different in the 9-epi alkaloids versus quinidine. This difference in positioning may determine the relative strengths, of the formation of hydrogen bonds with cellular constituents important to antimalarial activity and, therefore, may determine the relative strength of antimalarial activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttle G. A., Henry T. A., Solomon W., Trevan J. W., Gibbs E. M. The action of the cinchona and certain other alkaloids in bird malaria. III. Biochem J. 1938 Jan;32(1):47–58. doi: 10.1042/bj0320047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. D., Timony G., Fleckenstein L. The disposition of quinine in the rat isolated perfused liver: effect of dose size. J Pharm Pharmacol. 1990 Jan;42(1):26–29. doi: 10.1111/j.2042-7158.1990.tb05343.x. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty R., Benson W. R., Maienthal M., Stewart J. M. Crystal and molecular structure of quinidine. J Pharm Sci. 1978 Dec;67(12):1698–1701. doi: 10.1002/jps.2600671217. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Karle J. Anomalous dispersion of sulfur in quinidine sulfate, (C(20)H(25)N(2)O(2))(2)SO(4).2H(2)O: Implications for structure analysis. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5938–5941. doi: 10.1073/pnas.78.10.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle J. M., Karle I. L. Crystal structure and molecular structure of mefloquine methylsulfonate monohydrate: implications for a malaria receptor. Antimicrob Agents Chemother. 1991 Nov;35(11):2238–2245. doi: 10.1128/aac.35.11.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhous W. K., Gerena L., Kyle D. E., Oduola A. M. In vitro strategies for circumventing antimalarial drug resistance. Prog Clin Biol Res. 1989;313:61–72. [PubMed] [Google Scholar]
- Milhous W. K., Weatherly N. F., Bowdre J. H., Desjardins R. E. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob Agents Chemother. 1985 Apr;27(4):525–530. doi: 10.1128/aac.27.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduola A. M., Weatherly N. F., Bowdre J. H., Desjardins R. E. Plasmodium falciparum: cloning by single-erythrocyte micromanipulation and heterogeneity in vitro. Exp Parasitol. 1988 Jun;66(1):86–95. doi: 10.1016/0014-4894(88)90053-7. [DOI] [PubMed] [Google Scholar]
- Phillips R. E., Warrell D. A., White N. J., Looareesuwan S., Karbwang J. Intravenous quinidine for the treatment of severe falciparum malaria. Clinical and pharmacokinetic studies. N Engl J Med. 1985 May 16;312(20):1273–1278. doi: 10.1056/NEJM198505163122001. [DOI] [PubMed] [Google Scholar]
- Taggart J. V., Earle D. P., Berliner R. W., Zubrod C. G., Welch W. J., Wise N. B., Schroeder E. F., London I. M., Shannon J. A. STUDIES ON THE CHEMOTHERAPY OF THE HUMAN MALARIAS. III. THE PHYSIOLOGICAL DISPOSITION AND ANTIMALARIAL ACTIVITY OF THE CINCHONA ALKALOIDS. J Clin Invest. 1948 May;27(3 Pt 2):80–86. doi: 10.1172/JCI101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche D. L., Black J. A comparison of the antimalarial activity of the cinchona alkaloids against Plasmodium falciparum in vitro. J Trop Med Hyg. 1990 Jun;93(3):153–159. [PubMed] [Google Scholar]
- White N. J., Looareesuwan S., Warrell D. A., Chongsuphajaisiddhi T., Bunnag D., Harinasuta T. Quinidine in falciparum malaria. Lancet. 1981 Nov 14;2(8255):1069–1071. doi: 10.1016/s0140-6736(81)91275-7. [DOI] [PubMed] [Google Scholar]


