Abstract
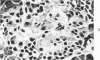
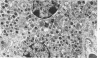
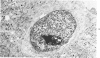
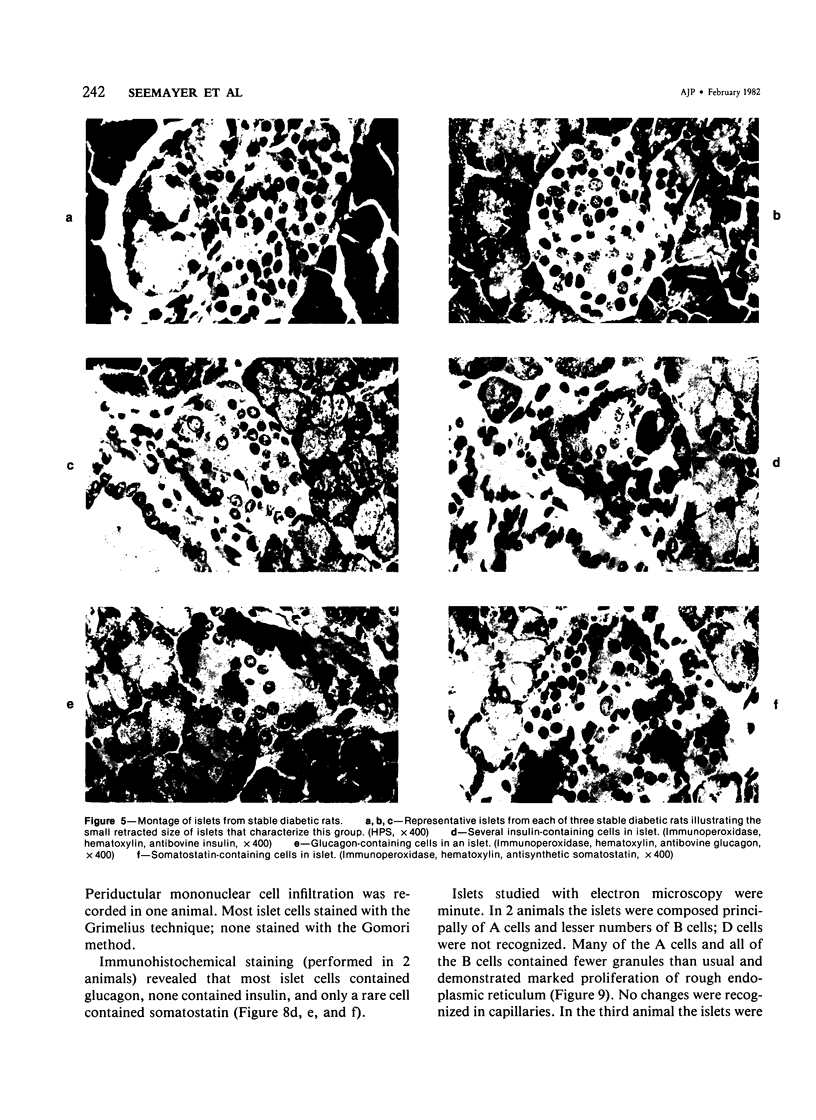
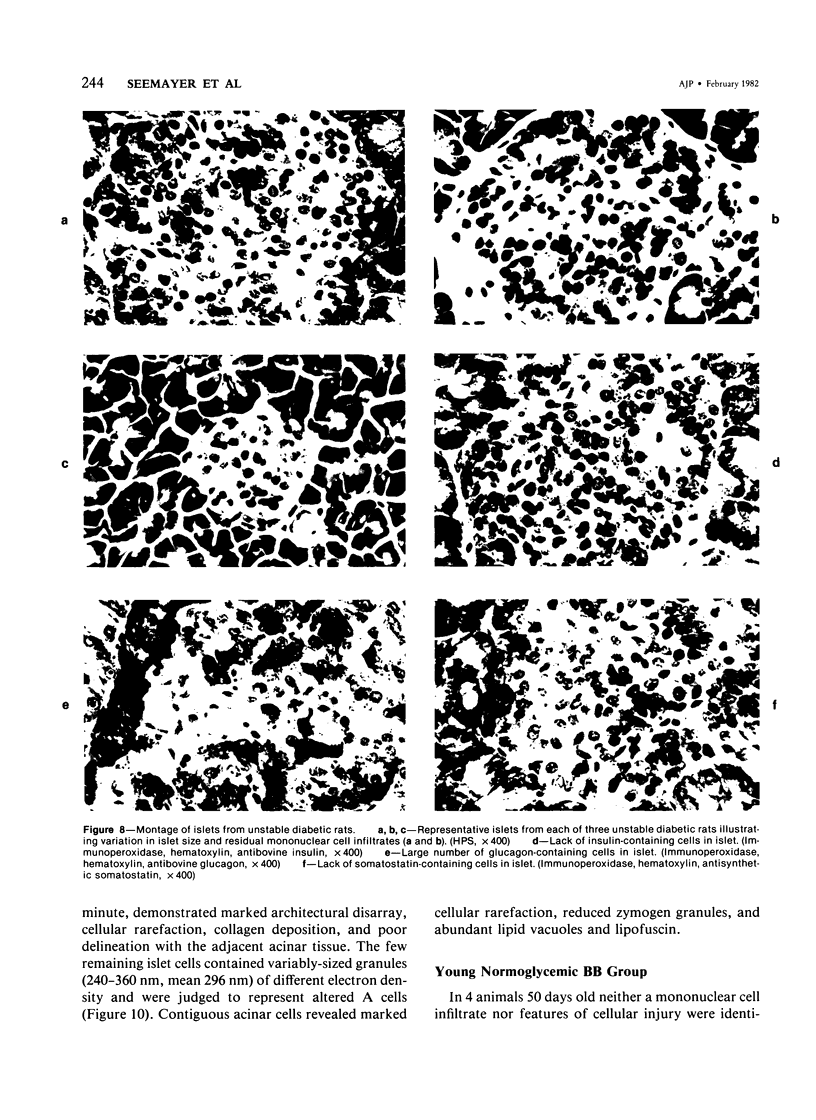
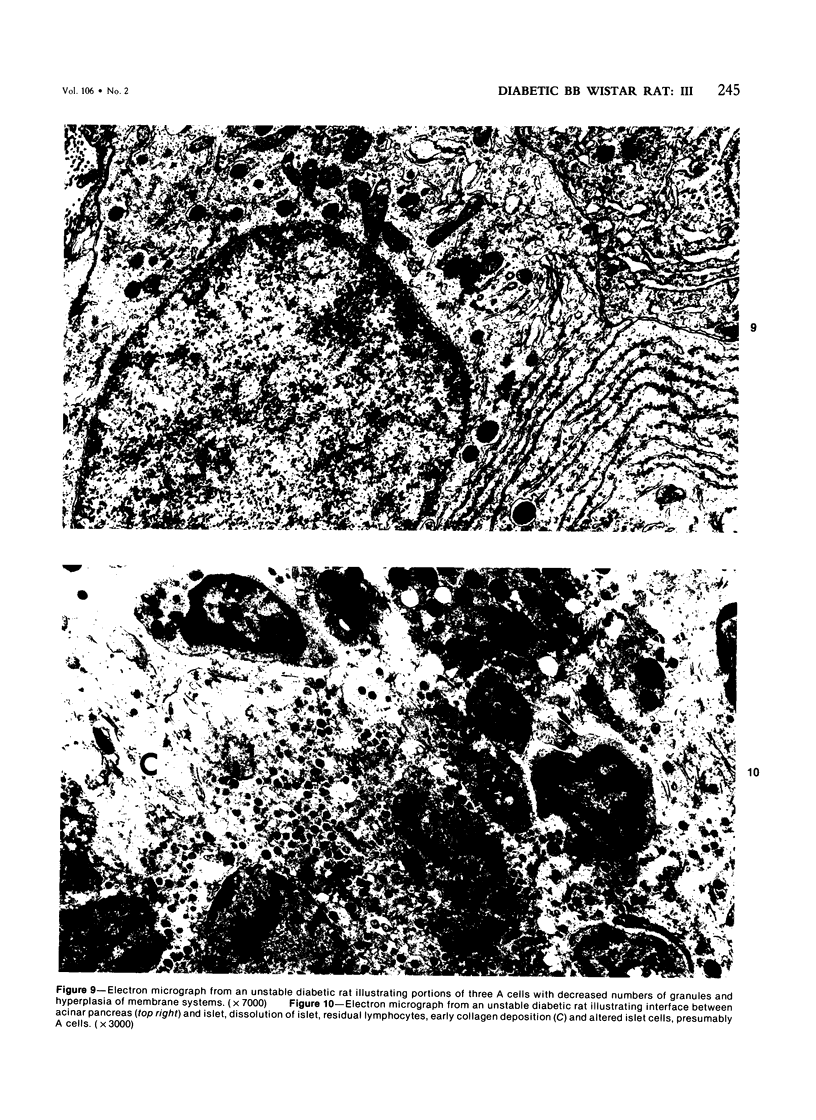
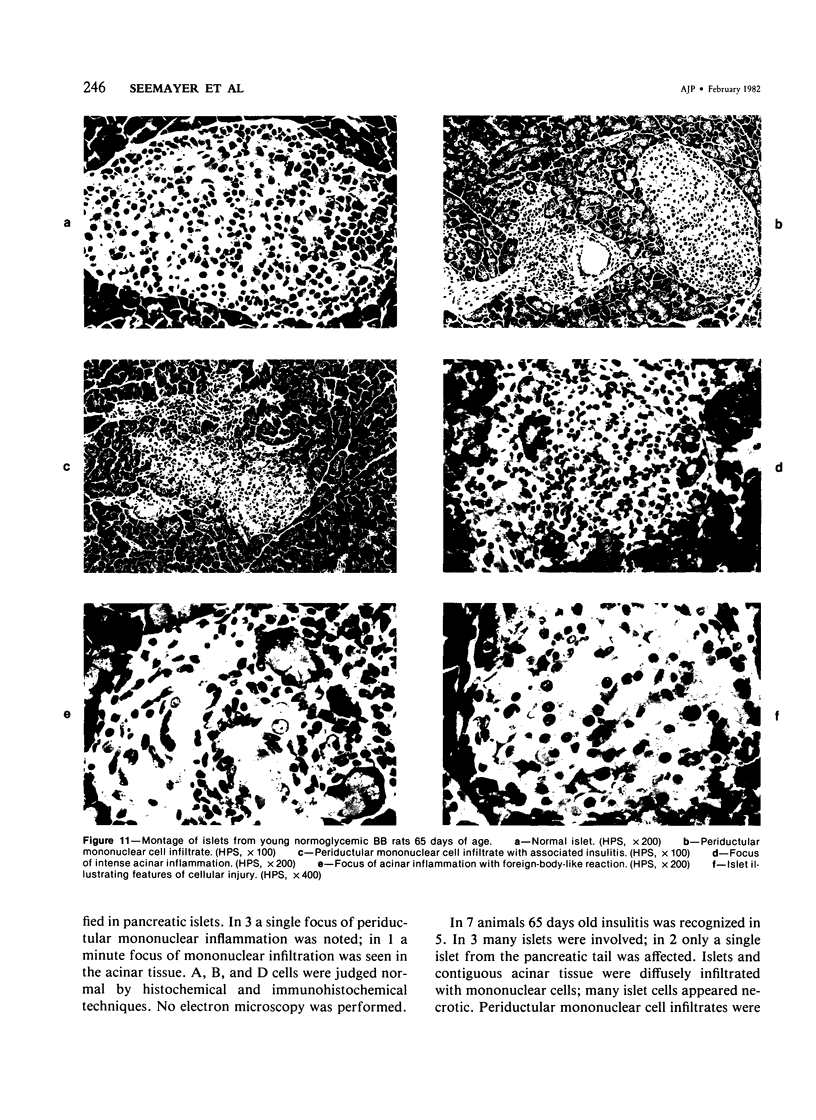
The pancreatic islet alterations were studied in spontaneously diabetic BB Wistar rats and in young (50 and 65 days old) normoglycemic BB rats with the use of light microscopy, immunohistochemistry, and electron microscopy. Three groups of diabetic rats were delineated: 1) early diabetes (1-3 days after detection of glycosuria), 2) stable diabetes (41-63 days after detection), and 3) unstable diabetes (7-22 days after detection). In early diabetes islets were extensively infiltrated by "activated" lymphocytes and macrophages, and B cells demonstrated marked degranulation, injury, and necrosis. Although no consistent changes were recorded in A cells, D cells appeared to be decreased in number. In stable and unstable diabetes, islets were small and markedly depleted of B cells, although more insulin-containing cells were identified in the stable group. The number of A and D cells appeared normal in the stable group, although some A cells appeared altered ultrastructurally. In the unstable group both A and D cells appeared decreased, and ultrastructurally altered A cells were again noted. These findings suggest that although B cells appear to be the principal islet target in this model, A and D cells also sustain cellular injury. Variable degrees of insulitis, B cell degranulation, and necrosis were documented in 65-day-old normoglycemic BB rats, suggesting that the destructive process in the islets is initiated well in advance of the onset of the clinical syndrome. The pancreases from many diabetic and normoglycemic BB rats also demonstrated mononuclear cell infiltrates distinct from insulitis in periductular and/or acinar locations. These infiltrates, not present in controls, appear to represent an additional morphologic expression of the process responsible for initiating the diabetic state.
Full text
PDF




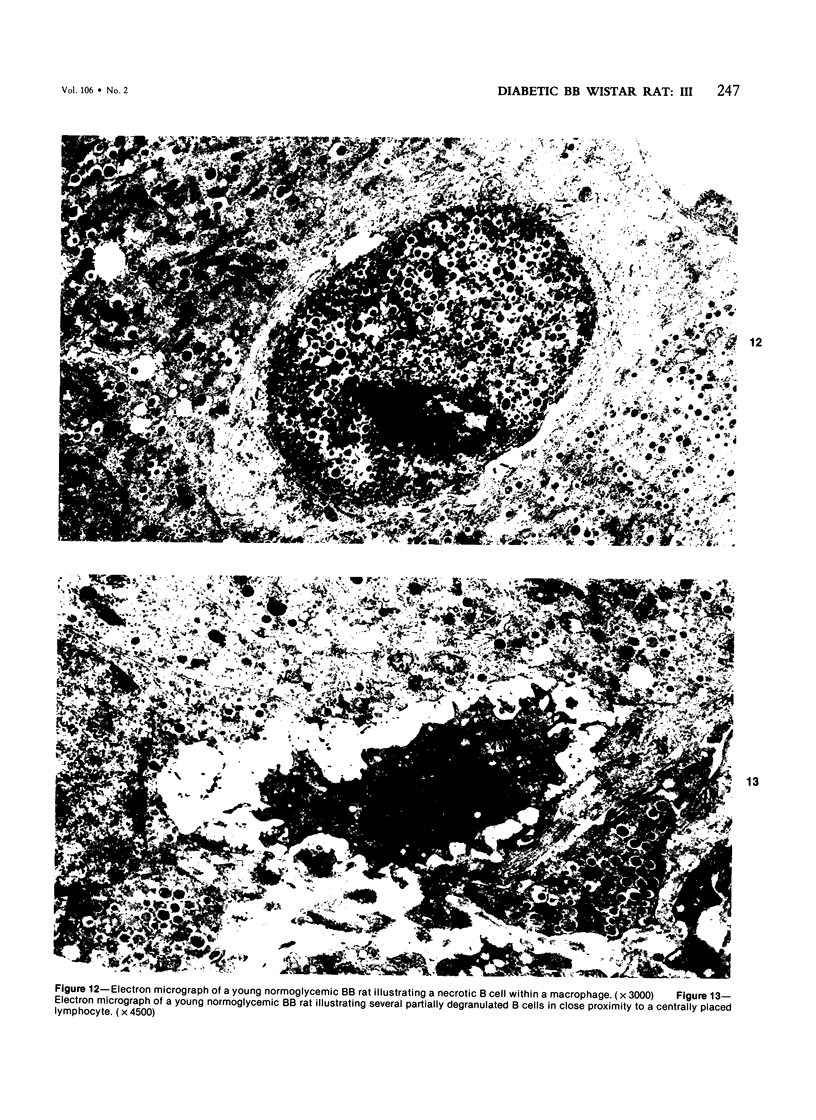


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craighead J. E., McLane M. F. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science. 1968 Nov 22;162(3856):913–914. doi: 10.1126/science.162.3856.913. [DOI] [PubMed] [Google Scholar]
- Erlandsen S. L., Parsons J. A., Burke J. P., Redick J. A., Van Orden D. E., Van Orden L. S. A modification of the unlabeled antibody enzyme method using heterologous antisera for the light microscopic and ultrastructural localization of insulin, glucagon and growth hormone. J Histochem Cytochem. 1975 Sep;23(9):666–677. doi: 10.1177/23.9.1176760. [DOI] [PubMed] [Google Scholar]
- GOMORI G. Aldehyde-fuchsin: a new stain for elastic tissue. Am J Clin Pathol. 1950 Jul;20(7):665–666. [PubMed] [Google Scholar]
- Gepts W., De Mey J. Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes. 1978;27 (Suppl 1):251–261. doi: 10.2337/diab.27.1.s251. [DOI] [PubMed] [Google Scholar]
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965 Oct;14(10):619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Grimelius L. A silver nitrate stain for alpha-2 cells in human pancreatic islets. Acta Soc Med Ups. 1968;73(5-6):243–270. [PubMed] [Google Scholar]
- Kalant N., Seemayer T. Malignant lymphoma in spontaneously diabetic rats. N Engl J Med. 1979 Mar 29;300(13):737–737. doi: 10.1056/NEJM197903293001318. [DOI] [PubMed] [Google Scholar]
- LECOMPTE P. M. Insulitis in early juvenile diabetes. AMA Arch Pathol. 1958 Oct;66(4):450–457. [PubMed] [Google Scholar]
- LeCompte P. M., Legg M. A. Insulitis (lymphocytic infiltration of pancreatic islets) in late-onset diabetes. Diabetes. 1972 Jun;21(6):762–769. doi: 10.2337/diab.21.6.762. [DOI] [PubMed] [Google Scholar]
- Like A. A., Appel M. C., Williams R. M., Rossini A. A. Streptozotocin-induced pancreatic insulitis in mice. Morphologic and physiologic studies. Lab Invest. 1978 Apr;38(4):470–486. [PubMed] [Google Scholar]
- Like A. A., Rossini A. A., Guberski D. L., Appel M. C., Williams R. M. Spontaneous diabetes mellitus: reversal and prevention in the BB/W rat with antiserum to rat lymphocytes. Science. 1979 Dec 21;206(4425):1421–1423. doi: 10.1126/science.388619. [DOI] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976 Jul 30;193(4251):415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Mordes J. P., Rossini A. A. Animal models of diabetes. Am J Med. 1981 Feb;70(2):353–360. doi: 10.1016/0002-9343(81)90772-5. [DOI] [PubMed] [Google Scholar]
- Nakhooda A. F., Like A. A., Chappel C. I., Murray F. T., Marliss E. B. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes. 1977 Feb;26(2):100–112. doi: 10.2337/diab.26.2.100. [DOI] [PubMed] [Google Scholar]
- Nakhooda A. F., Like A. A., Chappel C. I., Wei C. N., Marliss E. B. The spontaneously diabetic Wistar rat (the "BB" rat). Studies prior to and during development of the overt syndrome. Diabetologia. 1978 Mar;14(3):199–207. doi: 10.1007/BF00429781. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Wheatley T., Malaisse-Lagae F., Orci L. Elevated portal and peripheral blood concentration of immunoreactive somatostatin in spontaneously diabetic (BBL) Wistar rats: suppression with insulin. Diabetes. 1980 Sep;29(9):757–761. doi: 10.2337/diab.29.9.757. [DOI] [PubMed] [Google Scholar]
- Pelletier G., Leclerc R., Arimura A., Schally A. V. Letter: Immunohistochemical localization of somatostatin in the rat pancreas. J Histochem Cytochem. 1975 Sep;23(9):699–702. doi: 10.1177/23.9.1100709. [DOI] [PubMed] [Google Scholar]
- Rossini A. A., Williams R. M., Mordes J. P., Appel M. C., Like A. A. Spontaneous diabetes in the gnotobiotic BB/W rat. Diabetes. 1979 Nov;28(11):1031–1032. doi: 10.2337/diab.28.11.1031. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A., Oligny L. L., Tannenbaum G. S., Goldman H., Colle E. Animal model of human disease. Diabetes mellitus. Am J Pathol. 1980 Nov;101(2):485–488. [PMC free article] [PubMed] [Google Scholar]
- Stefan Y., Malaisse-Lagae F., Yoon J. W., Notkins A. L., Orci L. Virus-induced diabetes in mice: a quantitative evaluation of islet cell population by immunofluorescence technique. Diabetologia. 1978 Nov;15(5):395–401. doi: 10.1007/BF01219649. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Tannenbaum G. S., Colle E., Gurd W., Wanamaker L. Dynamic time-course studies of the spontaneously diabetic BB Wistar rat. I. Longitudinal profiles of plasma growth hormone, insulin, and glucose. Endocrinology. 1981 Dec;109(6):1872–1879. doi: 10.1210/endo-109-6-1872. [DOI] [PubMed] [Google Scholar]
- Tannenbaum G. S., Colle E., Wanamaker L., Gurd W., Goldman H., Seemayer T. A. Dynamic time-course studies of the spontaneously diabetic BB Wistar rat. II. Insulin-, glucagon-, and somatostatin-reactive cells in the pancreas. Endocrinology. 1981 Dec;109(6):1880–1887. doi: 10.1210/endo-109-6-1880. [DOI] [PubMed] [Google Scholar]