Abstract
The inflammatory modulating activity of specific prostaglandins has been examined for both immune and foreign-body types of pulmonary granulomas. Lung granuloma formation generated in mice by embolization of Schistosoma mansoni eggs was markedly suppressed by treatment with the stable, functional analog of prostaglandin E, (PGE1), (15-(S)-15-methyl PGE1) while treatment with PGF2 alpha augmented the granulomatous response. Despite marked effects on egg-induced granuloma formation, PGs had no significant effect on the foreign body lesion induced by Sephadex beads. Likewise, PGs had no effect on the primary antibody response to schistosome egg antigens. However, notable derangements in splenic lymphoid populations occurred. While T-cell numbers appeared constant in face of PG treatment, B-cell populations were depressed by methyl-PGE1 and augmented by PGF2 alpha. Further analysis revealed that methyl-PGE1 appeared to suppress both the induction and elicitation phases of the cell-mediated response to schistosome eggs. Cyclophosphamide treatment could partially reverse this suppression, but the induction of suppressor cell activity was not solely responsible for this effect. The possible role and mechanism of PGs as modulators of chronic inflammation is discussed.
Full text
PDF


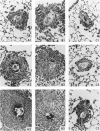
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonta I. L., Parnham M. J. Time-dependent stimulatory and inhibitory effects of prostaglandin E1 on exudative and tissue components of granulomatous inflammation in rats. Br J Pharmacol. 1979 Mar;65(3):465–472. doi: 10.1111/j.1476-5381.1979.tb07852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L. Schistosomiasis mansoni: a granulomatous disease of cell-mediated immune etiology. Ann N Y Acad Sci. 1976;278:36–46. doi: 10.1111/j.1749-6632.1976.tb47014.x. [DOI] [PubMed] [Google Scholar]
- COKER C. M., LICHTENBERG F. A revised method for isolation of Schistosoma mansoni eggs for biological experimentation. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):780–782. doi: 10.3181/00379727-92-22612. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. In vitro characterization of T lymphocyte subsets involved in the production and suppression of migration inhibition factor. J Exp Med. 1980 Jun 1;151(6):1398–1412. doi: 10.1084/jem.151.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Population dynamics of T and B lymphocytes in the lymphoid organs, circulation, and granulomas of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1979 Mar;28(2):291–299. doi: 10.4269/ajtmh.1979.28.291. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Dunn C. J., Willoughby D. A., Giroud J. P., Yamamoto S. An appraisal of the interrelationships between prostaglandins and cyclic nucleotides in inflammation. Biomedicine. 1976;24(4):214–220. [PubMed] [Google Scholar]
- Eardley D. D., Hugenberger J., McVay-Boudreau L., Shen F. W., Gershon R. K., Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978 Apr 1;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Kunkel S. L., Ward P. A., Zurier R. B. Suppression by prostaglandin E1 of vascular permeability induced by vasoactive inflammatory mediators. J Immunol. 1980 Dec;125(6):2591–2596. [PubMed] [Google Scholar]
- Friedman S. A., Remold-O'Donnell E., Piessens W. F. Enhanced PGE production by MAF-treated peritoneal exudate macrophages. Cell Immunol. 1979 Jan;42(1):213–218. doi: 10.1016/0008-8749(79)90237-5. [DOI] [PubMed] [Google Scholar]
- Fulton A. M., Levy J. G. The possible role of prostaglandins in mediating immune suppression by nonspecific T suppressor cells. Cell Immunol. 1980 Jun;52(1):29–37. doi: 10.1016/0008-8749(80)90397-4. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Kato K., Yamamoto K. Involvement of prostaglandin E1 in delayed-type hypersensitivity suppression induced with live Mycobacterium bovis BCG. Infect Immun. 1982 Apr;36(1):426–429. doi: 10.1128/iai.36.1.426-429.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Thrall R. S., Kunkel R. G., McCormick J. R., Ward P. A., Zurier R. B. Suppression of immune complex vasculitis in rats by prostaglandin. J Clin Invest. 1979 Nov;64(5):1525–1529. doi: 10.1172/JCI109611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., DeBernardo R. The immediate allergic response: in vitro action of cyclic AMP-active and other drugs on the two stages of histamine release. J Immunol. 1971 Oct;107(4):1131–1136. [PubMed] [Google Scholar]
- Mertin J., Stackpoole A. Prostaglandin precursors and the cell-mediated immune response. Cell Immunol. 1981 Aug;62(2):293–300. doi: 10.1016/0008-8749(81)90329-4. [DOI] [PubMed] [Google Scholar]
- Morley J. Prostaglandins and lymphokines in arthritis. Prostaglandins. 1974 Nov 25;8(4):315–326. doi: 10.1016/s0090-6980(74)80096-1. [DOI] [PubMed] [Google Scholar]
- Ohuchi K., Sato H., Tsurufuji S. The content of prostaglandin E and prostaglandin F2alpha in the exudate of carrageenin granuloma of rats. Biochim Biophys Acta. 1976 Mar 26;424(3):439–448. doi: 10.1016/0005-2760(76)90033-3. [DOI] [PubMed] [Google Scholar]
- Quagliata F., Lawrence V. J., Phillips-Quagliata J. M. Prostaglandin E 1 as a regulator of lymphocyte function. Selective action on B lymphocytes and synergy with procarbazine in depression of immune responses. Cell Immunol. 1973 Mar;6(3):457–465. doi: 10.1016/0008-8749(73)90044-0. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER M. IMMUNE LYSIS OF THYMUS AND SPLEEN CELLS OF EMBRYONIC AND NEONATAL MICE. J Immunol. 1965 Mar;94:358–364. [PubMed] [Google Scholar]
- Schwartz A., Askenase P. W., Gershon R. K. Regulation of delayed-type hypersensitivity reactions by cyclophosphamide-sensitive T cells. J Immunol. 1978 Oct;121(4):1573–1577. [PubMed] [Google Scholar]
- Snyder D. S., Lu C. Y., Unanue E. R. Control of macrophage Ia expression in neonatal mice--role of a splenic suppressor cell. J Immunol. 1982 Mar;128(3):1458–1465. [PubMed] [Google Scholar]
- Warren K. S. A functional classification of granulomatous inflammation. Ann N Y Acad Sci. 1976;278:7–18. doi: 10.1111/j.1749-6632.1976.tb47011.x. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Nowowiejski I. Mitogen-induced changes in lymphocyte prostaglandin levels: a signal for the induction of suppressor cell activity. Cell Immunol. 1978 Nov;41(1):72–85. doi: 10.1016/s0008-8749(78)80029-x. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Quagliata F. Effect of prostaglandin E 1 on adjuvant arthritis. Nature. 1971 Dec 3;234(5327):304–305. doi: 10.1038/234304a0. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]