Abstract
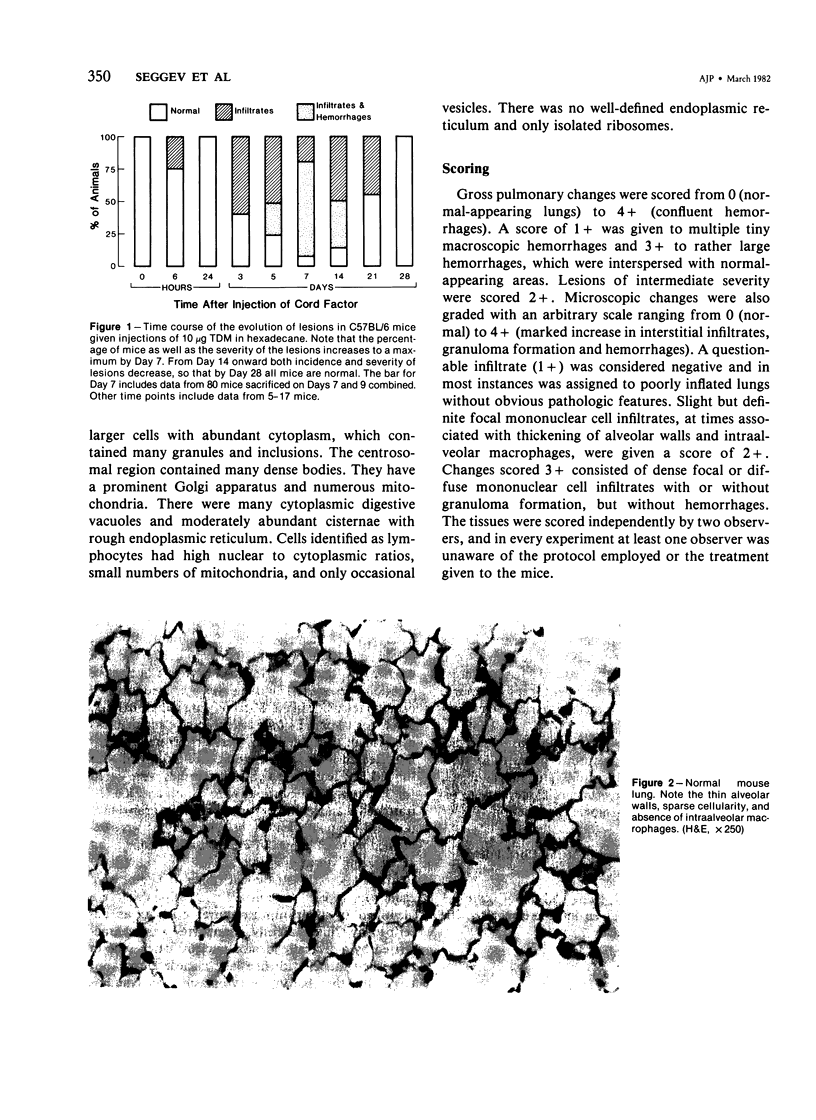
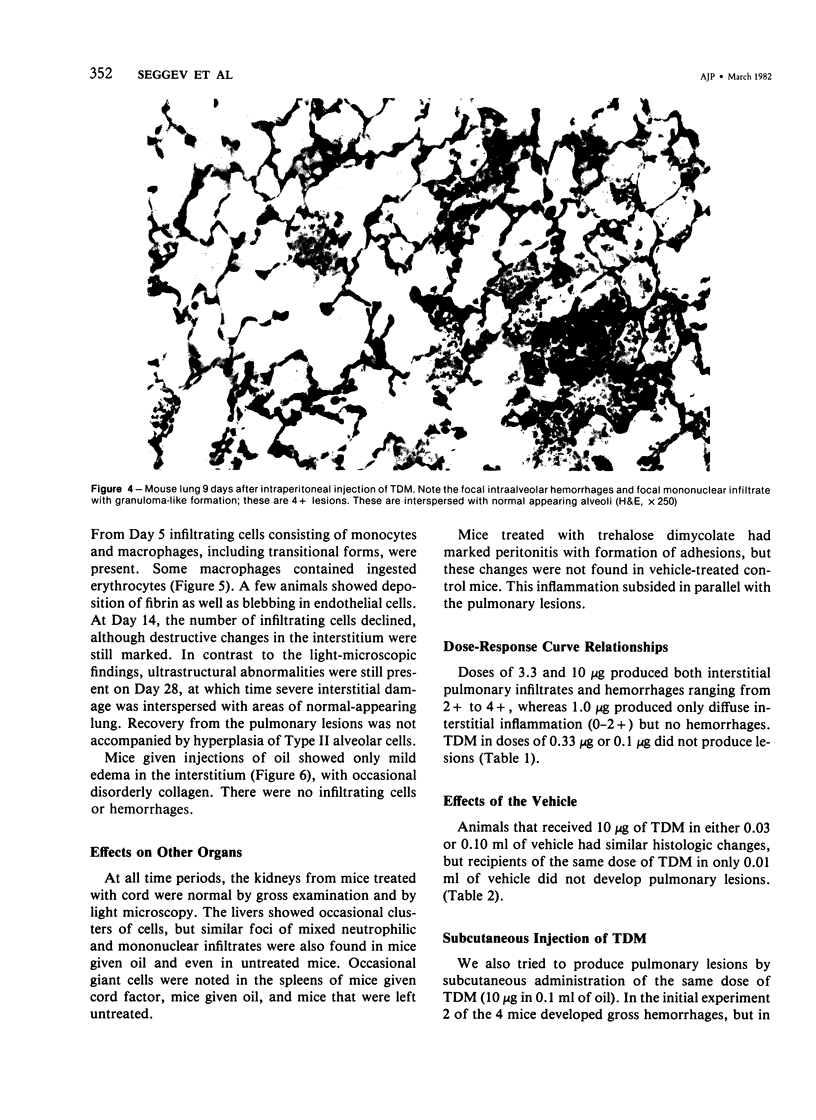
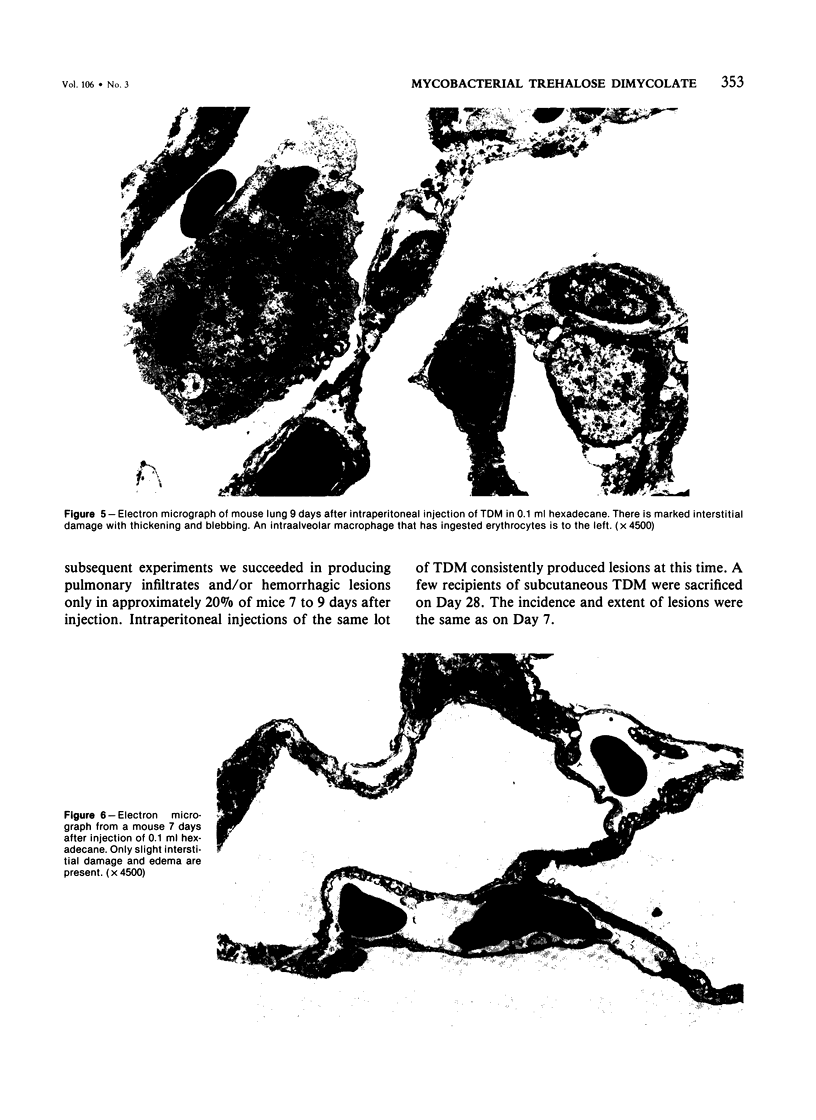
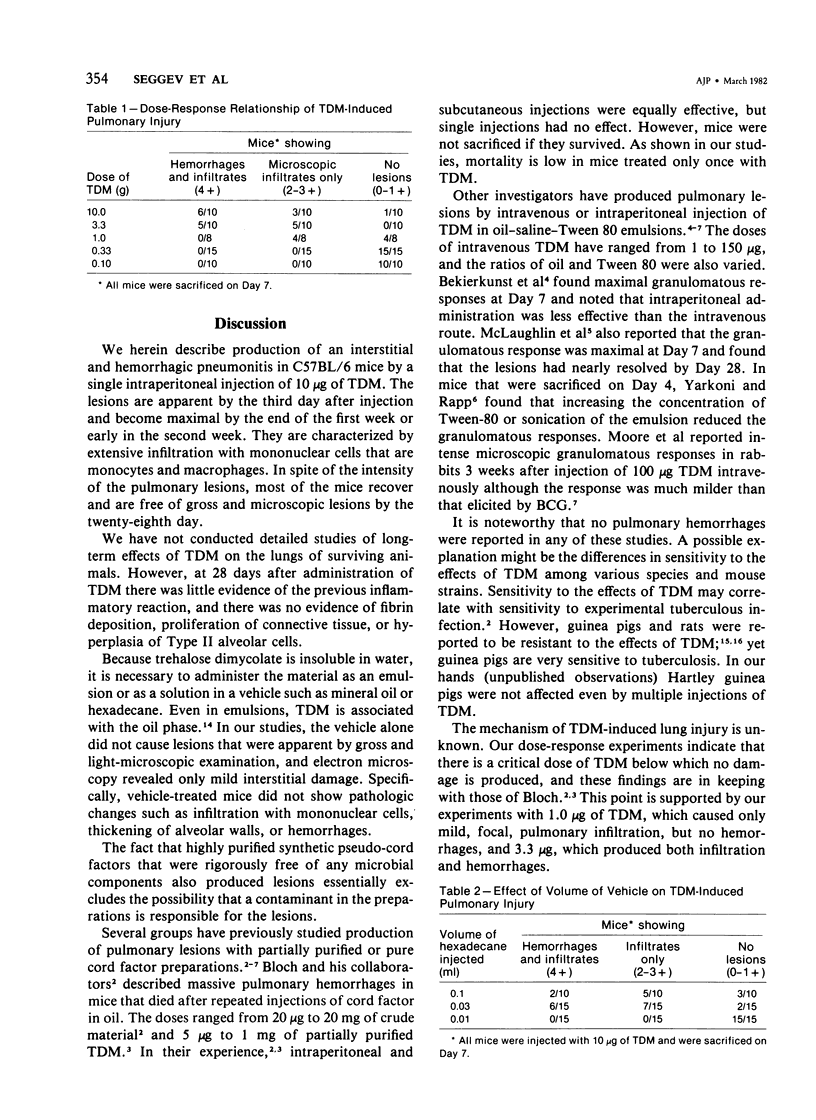
Intraperitoneal injections of cord factor (trehalose dimycolate, TDM) provides a model for interstitial and hemorrhagic lung disease that is produced by a chemically defined substance. A single injection of 10 micrograms of TDM, in light mineral oil or hexadecane, into C57BL/6 mice produces interstitial and hemorrhagic pneumonitis. Following injection of TDM the pulmonary lesions increase gradually and become maximal by the seventh to ninth day, at which time 70% of the mice show both gross hemorrhages and dense mononuclear infiltrates; an additional 20% of the mice show only microscopic lesions. From day 14 onward the incidence and severity of the lesions decrease, and by day 28 the lungs are normal by both gross and light-microscopy examination. Only 5% of the mice succumb. Except for peritonitis other organs are not affected. Doses of 3.3 and 10 micrograms of TDM are equally effective in producing the lesions, but a dose of 1.0 microgram of TDM causes only mild interstitial inflammation and lesser doses do not induce lesions. A single subcutaneous injection of 10 micrograms of TDM causes lesions in only 20% of mice. Vehicle-injected mice do not develop lesions. Electron microscopy revealed that the majority of the infiltrating cells are monocytes and macrophages and that extensive interstitial damage is produced. The mechanism of the effects of TDM are unknown and is currently under study. Our preliminary data suggests that the phenomenon is dependent upon T-lymphocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Petit J. F., Lederer E. Isolation and properties of a macromolecular, water-soluble, immuno-adjuvant fraction from the cell wall of Mycobacterium smegmatis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):851–854. doi: 10.1073/pnas.69.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCH H., SORKIN E., ERLENMEYER H. A toxic lipid component of the tubercle bacillus (cord factor). I. Isolation from petroleum ether extracts of young bacterial cultures. Am Rev Tuberc. 1953 May;67(5):629–643. doi: 10.1164/art.1953.67.5.629. [DOI] [PubMed] [Google Scholar]
- BLOCH H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950 Feb;91(2):197-218, pl. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E., Vilkas E., Adam A., Lederer E. Granuloma formation induced in mice by chemically defined mycobacterial fractions. J Bacteriol. 1969 Oct;100(1):95–102. doi: 10.1128/jb.100.1.95-102.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., Jiang K. S. Pseudo cord factors: derivatives of alpha-D-glucopyranuronosyl (1-1) alpha-D-glucopyranuronoside. Chem Phys Lipids. 1979 Oct;25(2):209–224. doi: 10.1016/0009-3084(79)90069-0. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Yamamoto K. I., Ribi E. Delayed hypersensitivity and granulomatous response after immunization with protein antigens associated with a mycobacterial glycolipid and oil droplets. J Immunol. 1976 Feb;116(2):482–488. [PubMed] [Google Scholar]
- Kato M., Fukushi K. Studies of a biochemical lesion in experimental tuberculosis in mice. X. Mitochondrial swelling induced by cord factor in vivo and accompanying biochemical change. Am Rev Respir Dis. 1969 Jul;100(1):42–46. doi: 10.1164/arrd.1969.100.1.42. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. A., Parker R., Hadlow W. J., Toubiana R., Ribi E. Moieties of mycobacterial mycolates required for inducing granulomatous reactions. Cell Immunol. 1978 Jun;38(1):14–24. doi: 10.1016/0008-8749(78)90027-8. [DOI] [PubMed] [Google Scholar]
- Moore V. L., Myrvik Q. N., Kato M. Role of cord factor (trehalose-6,6'-dimycolate) in allergic granuloma formation in rabbits. Infect Immun. 1972 Jul;6(1):5–8. doi: 10.1128/iai.6.1.5-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLL H. The chemistry of some native constituents of the purified wax of Mycobacterium tuberculosis. J Biol Chem. 1957 Jan;224(1):149–164. [PubMed] [Google Scholar]
- Ofek I., Bekierkunst A. Chemotactic response of leukocytes to cord factor (trehalose-6,6'-dimycolate). J Natl Cancer Inst. 1976 Dec;57(6):1379–1381. doi: 10.1093/jnci/57.6.1379. [DOI] [PubMed] [Google Scholar]
- Ramanathan V. D., Curtis J., Turk J. L. Activation of the alternative pathway of complement by mycobacteria and cord factor. Infect Immun. 1980 Jul;29(1):30–35. doi: 10.1128/iai.29.1.30-35.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R., Tanaka A., Sugiyama K. Cord factor not toxic in rats. Am Rev Respir Dis. 1975 Oct;112(4):578–580. doi: 10.1164/arrd.1975.112.4.578. [DOI] [PubMed] [Google Scholar]
- Tenu J. P., Lederer E., Petit J. F. Stimulation of thymocyte mitogenic protein secretion and of cytostatic activity of mouse peritoneal macrophages by trehalose dimycolate and muramyldipeptide. Eur J Immunol. 1980 Aug;10(8):647–653. doi: 10.1002/eji.1830100813. [DOI] [PubMed] [Google Scholar]
- Voith M. A., Lichtenfeld K. M., Schimpff S. C., Wiernik P. H. Systemic complications of MER immunotherapy of cancer: pulmonary granulomatosis and rash. Cancer. 1979 Feb;43(2):500–504. doi: 10.1002/1097-0142(197902)43:2<500::aid-cncr2820430215>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Yarkoni E., Rapp H. J. Granuloma formation in lungs of mice after intravenous administration of emulsified trehalose-6,6'-dimycolate (cord factor): reaction intensity depends on size distribution of the oil droplets. Infect Immun. 1977 Nov;18(2):552–554. doi: 10.1128/iai.18.2.552-554.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni E., Wang L., Bekierkunst A. Stimulation of macrophages by cord factor and by heat-killed and living BCG. Infect Immun. 1977 Apr;16(1):1–8. doi: 10.1128/iai.16.1.1-8.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]







