Abstract
Pancreatic ectasia (PE) is a common incidental finding in people dying from uremia. It has been described as dilatation of acini, inspissation of secretions, and proliferation of ductal cells. PE occurred in 17 macaques, 11 of which were known to have been uremic. The lesion was studied by light and electronmicroscopy and histochemistry and by construction of a three-dimensional model of a dilated acinar ductal system from serial semithick Epon sections. Atrophic acinar cells interspersed with clumps of centroacinar cells lined all portions of the system. There was no evidence of ductular proliferation. Fibrillar material was present in the dilated acinar lumens and associated with epithelial cells and leukocytes, but no blockage of the system was demonstrated. The lesion is similar to those induced by a variety of experimental procedures and to the pancreatic lesions of cystic fibrosis.
Full text
PDF


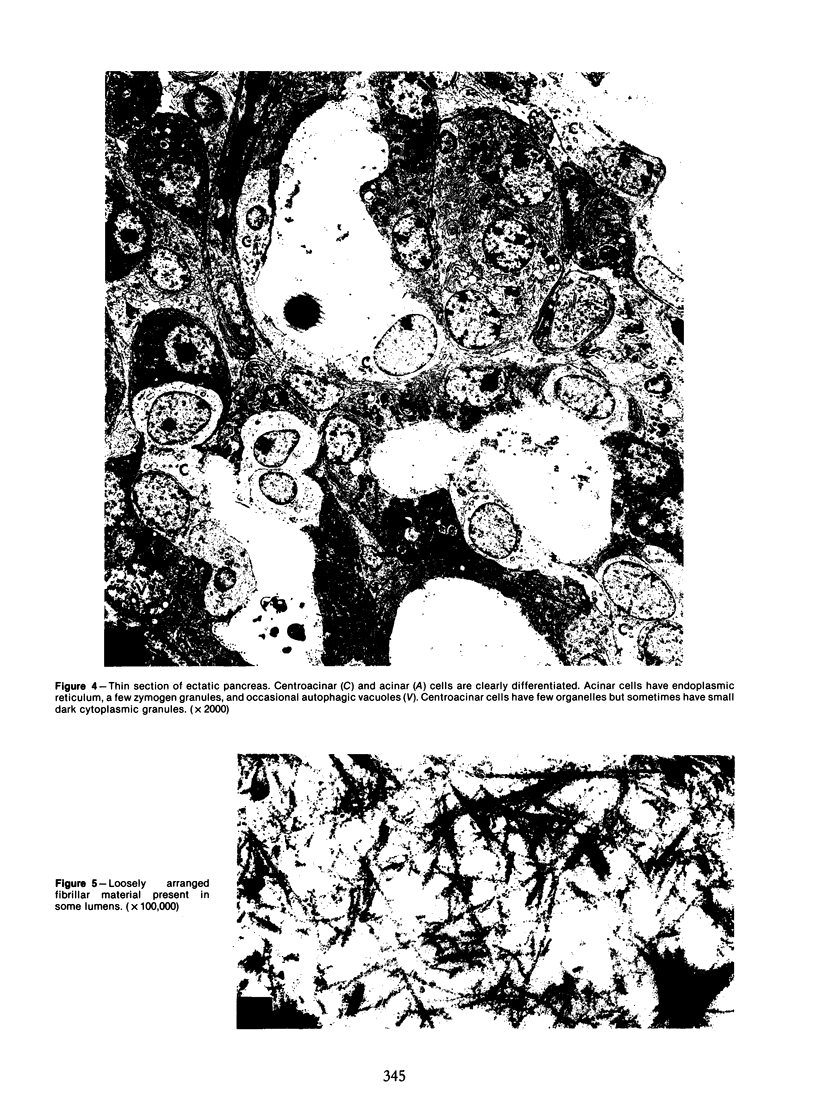


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAAR H. S. Fibrocystic disease of the pancreas. Lancet. 1953 Jul 11;265(6776):80–83. doi: 10.1016/s0140-6736(53)91101-5. [DOI] [PubMed] [Google Scholar]
- BALL W. P., BAGGENSTOSS A. H., BARGEN J. A. Pancreatic lesions associated with chronic ulcerative colitis. Arch Pathol (Chic) 1950 Sep;50(3):347–358. [PubMed] [Google Scholar]
- Bockman D. E. Anastomosing tubular arrangement of the exocrine pancreas. Am J Anat. 1976 Sep;147(1):113–118. doi: 10.1002/aja.1001470111. [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Black O., Jr, Mills L. R., Webster P. D. Origin of tubular complexes developing during induction of pancreatic adenocarcinoma by 7,12-dimethylbenz(a)anthracene. Am J Pathol. 1978 Mar;90(3):645–658. [PMC free article] [PubMed] [Google Scholar]
- Bolender R. P. Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J Cell Biol. 1974 May;61(2):269–287. doi: 10.1083/jcb.61.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquist L., Edström C. Ultrastructure of pancreatic acinar and islet parenchyma in rats at various intervals after duct ligation. Virchows Arch A Pathol Pathol Anat. 1970;349(1):69–79. doi: 10.1007/BF00548522. [DOI] [PubMed] [Google Scholar]
- Boquist L. The effect of excess methionine on the pancreas. A light and electron microscopic study in the Chinese hamster with particular reference to degenerative changes. Lab Invest. 1969 Aug;21(2):96–104. [PubMed] [Google Scholar]
- Churg A., Richter W. R. Early changes in the exocrine pancreas of the dog and rat after ligation of the pancreatic duct. A light and electron microscopic study. Am J Pathol. 1971 Jun;63(3):521–546. [PMC free article] [PubMed] [Google Scholar]
- HERMAN L., FITZGERALD P. J. Restitution of pancreatic acinar cells following ethionine. J Cell Biol. 1962 Feb;12:297–312. doi: 10.1083/jcb.12.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEV R., SPICER S. S. A HISTOCHEMICAL COMPARISON OF HUMAN EPITHELIAL MUCINS IN NORMAL AND IN HYPERSECRETORY STATES INCLUDING PANCREATIC CYSTIC FIBROSIS. Am J Pathol. 1965 Jan;46:23–47. [PMC free article] [PubMed] [Google Scholar]
- Norris R. F., Tyson R. M. The Pathogenesis of Polycystic Pancreas: Reconstruction of Cystic Elements in One Case. Am J Pathol. 1947 May;23(3):485–499. [PMC free article] [PubMed] [Google Scholar]
- PORTA E. A., STEIN A. A., PATTERSON P. ULTRASTRUCTURAL CHANGES OF THE PANCREAS AND LIVER IN CYSTIC FIBROSIS. Am J Clin Pathol. 1964 Nov;42:451–465. doi: 10.1093/ajcp/42.5.451. [DOI] [PubMed] [Google Scholar]
- POWERS S. R., Jr, STEIN A. A. Pancreatic-acinar ectasia. AMA Arch Pathol. 1956 Dec;62(6):494–496. [PubMed] [Google Scholar]
- WALTERS M. N., GIBB D. G. CYSTIC FIBROSIS AND ACINAR ECTASIA OF THE PANCREAS: HISTOCHEMICAL COMPARISON OF THE INTRALUMINAL SECRETIONS. J Pathol Bacteriol. 1965 Jan;89:89–93. [PubMed] [Google Scholar]
- WALTERS M. N. STUDIES ON THE EXOCRINE PANCREAS. I. NONSPECIFIC PANCREATIC DUCTULAR ECTASIA. Am J Pathol. 1964 Jun;44:973–981. [PMC free article] [PubMed] [Google Scholar]
- WARREN S., SOMMERS S. C. Pathogenesis of ulcerative colitis. Am J Pathol. 1949 Jul;25(4):657–679. [PMC free article] [PubMed] [Google Scholar]
- ZELANDER T., EKHOLM R., EDLUND Y. THE ULTRASTRUCTURE OF THE RAT EXOCRINE PANCREAS AFTER EXPERIMENTALLY OCCLUDED OUTFLOW. J Ultrastruct Res. 1964 Feb;10:89–102. doi: 10.1016/s0022-5320(64)90023-1. [DOI] [PubMed] [Google Scholar]
- ZELANDER T., EKHOLM R., EDLUND Y. The ultrastructural organization of the rat exocrine pancreas. III. Intralobular vessels and nerves. J Ultrastruct Res. 1962 Aug;7:84–101. doi: 10.1016/s0022-5320(62)80029-x. [DOI] [PubMed] [Google Scholar]
- Zeligs J. D., Janoff A., Dumont A. E. The course and nature of acinar cell death following pancreatic ligation in the guinea pig. Am J Pathol. 1975 Aug;80(2):203–226. [PMC free article] [PubMed] [Google Scholar]