Abstract
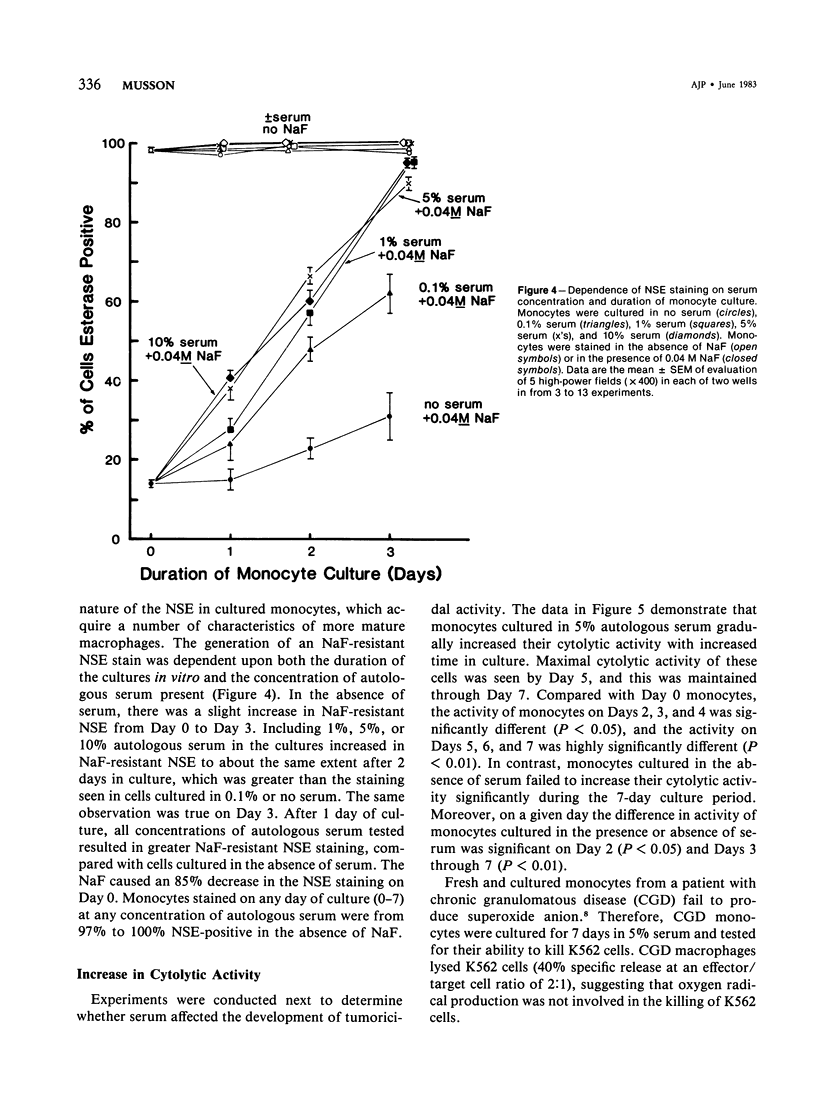
The dependence of human monocyte maturation in vitro on autologous serum was examined. If autologous serum was present during the monocyte culture, cytolysis of K562 target cells increased, intracellular levels of three lysosomal enzymes increased, and the fluoride-inhibitable esterase staining of the monocytes changed into a fluoride-resistant esterase stain (characteristic of more mature extravascular mononuclear phagocytes). Monocytes cultured in the presence and in the absence of serum also assumed different shapes. All of these changes were dependent on the concentration of autologous serum present (0-10%) and the length of time the monocytes were in culture (0-7 days). Lack of development by monocytes cultured in the absence of serum was not due to a general loss of the ability of these cells to function, because phagocytosis of antibody-coated erythrocytes was not lost following 7 days in culture in the absence of serum.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Eden A., Cohn Z. A. The induction of macrophage spreading: role of coagulation factors and the complement system. J Exp Med. 1976 Dec 1;144(6):1531–1544. doi: 10.1084/jem.144.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech M. J., Bainton D. F. Identification of alpha-naphthyl butyrate esterase as a plasma membrane ectoenzyme of monocytes and as a discrete intracellular membrane-bounded organelle in lymphocytes. J Exp Med. 1981 Jan 1;153(1):182–195. doi: 10.1084/jem.153.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D. J., Churchill W. H. Cytotoxicity of human macrophages for tumor cells: enhancement by bacterial lipopolysaccharides (LPS). J Immunol. 1980 Feb;124(2):708–712. [PubMed] [Google Scholar]
- Chapman H. A., Jr, Hibbs J. B., Jr Modulation of macrophage tumoricidal capability by components of normal serum: a central role for lipid. Science. 1977 Jul 15;197(4300):282–285. doi: 10.1126/science.195338. [DOI] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D. G., Hubbard W. J., Koren H. S. Tumor cell killing by freshly isolated peripheral blood monocytes. Cell Immunol. 1981 Mar 1;58(2):426–435. doi: 10.1016/0008-8749(81)90235-5. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Hammerstrøm J. Human monocyte-mediated cytotoxicity to K-562 cells: activation by lymphokines. Scand J Immunol. 1979;10(6):575–584. doi: 10.1111/j.1365-3083.1979.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Hammerstrøm J. In vitro influence of endotoxin on human mononuclear phagocyte structure and function. 2. Enhancement of the expression of cytoststic and cytolytic activity of normal and lymphokine-activated monocytes. Acta Pathol Microbiol Scand C. 1979 Dec;87(6):391–399. [PubMed] [Google Scholar]
- Hammerstrøm J., Unsgaard G. In vitro influence of endotoxin on human mononuclear phagocyte structure and function. 1. Depression of protein synthesis, phagocytosis of Candida albicans and induction of cytostatic activity. Acta Pathol Microbiol Scand C. 1979 Dec;87(6):381–389. [PubMed] [Google Scholar]
- Hibbs J. B., Jr Heterocytolysis by macrophages activated by bacillus Calmette-Guérin: lysosome exocytosis into tumor cells. Science. 1974 Apr 26;184(4135):468–471. doi: 10.1126/science.184.4135.468. [DOI] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarbre P., Hoidal J., Vesella R., Rinehart J. Human pulmonary macrophage tumor cell cytotoxicity. Blood. 1980 Apr;55(4):612–617. [PubMed] [Google Scholar]
- Leonard E. J., Skeel A. H. Isolation of macrophage stimulating protein (MSP) from human serum. Exp Cell Res. 1978 Jun;114(1):117–126. doi: 10.1016/0014-4827(78)90043-5. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Bar Shavit Z., Peri G., Polentarutti N., Bordignon C., Sessa C., Mangioni C. Natural cytotoxicity on tumour cells of human macrophages obtained from diverse anatomical sites. Clin Exp Immunol. 1980 Mar;39(3):776–784. [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Edelson P. J., Gass K. Changes in macrophage ectoenzymes associated with anti-tumor activity. J Immunol. 1980 Sep;125(3):1312–1317. [PubMed] [Google Scholar]
- Musson R. A., Becker E. L. The inhibitory effect of chemotactic factors on erythrophagocytosis by human neutrophils. J Immunol. 1976 Aug;117(2):433–439. [PubMed] [Google Scholar]
- Musson R. A., McPhail L. C., Shafran H., Johnston R. B., Jr Differences in the ability of human peripheral blood monocytes and in vitro monocyte-derived macrophages to produce superoxide anion: studies with cells from normals and patients with chronic granulomatous disease. J Reticuloendothel Soc. 1982 Mar;31(3):261–266. [PubMed] [Google Scholar]
- Musson R. A., Shafran H., Henson P. M. Intracellular levels and stimulated release of lysosomal enzymes from human peripheral blood monocytes and monocyte-derived macrophages. J Reticuloendothel Soc. 1980 Sep;28(3):249–264. [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Musson R. A., Henson P. M. Development of functional complement receptors during in vitro maturation of human monocytes into macrophages. J Immunol. 1980 Nov;125(5):2236–2244. [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Rinehart J. J., Orser M., Kaplan M. E. Human monocyte and macrophage modulation of lymphocyte proliferation. Cell Immunol. 1979 Apr;44(1):131–143. doi: 10.1016/0008-8749(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Rinehart J. J., Vessella R., Lange P., Kaplan M. E., Gormus B. J. Characterization and comparison of human monocyte- and macrophage-induced tumor cell cytotoxicity. J Lab Clin Med. 1979 Mar;93(3):361–369. [PubMed] [Google Scholar]
- Schmalzl F., Braunsteiner H. The cytochemistry of monocytes and macrophages. Ser Haematol. 1970;3(2):93–131. [PubMed] [Google Scholar]
- Sundsmo J. S., Götze O. Human monocyte spreading induced by factor B of the alternative pathway of complement activation. Cell Immunol. 1980 Jun;52(1):1–17. doi: 10.1016/0008-8749(80)90395-0. [DOI] [PubMed] [Google Scholar]
- Wiener E., Levanon D. The in vitro interaction between bacterial lipopolysaccharide and differentiating monocytes. Lab Invest. 1968 Dec;19(6):584–590. [PubMed] [Google Scholar]
- Yatziv S., Epstein L. B., Epstein C. J. Monocyte-derived macrophages: an in vitro system for studying hereditary lysosomal storage diseases. Pediatr Res. 1978 Sep;12(9):939–944. doi: 10.1203/00006450-197809000-00011. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Grusky G., Marcus A. Transformation of monocytes into "fat" cells. Lab Invest. 1978 May;38(5):620–628. [PubMed] [Google Scholar]
- Zuckerman S. H., Ackerman S. K., Douglas S. D. Long-term human peripheral blood monocyte cultures: establishment, metabolism and morphology of primary human monocyte-macrophage cell cultures. Immunology. 1979 Oct;38(2):401–411. [PMC free article] [PubMed] [Google Scholar]