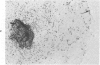
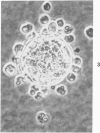
Abstract
In vitro growth patterns and morphologic characteristics of five low-grade human papillary transitional cell carcinomas (TCCs) were compared and contrasted with those of normal human urothelial cells in culture. Biopsies of TCC were performed by transurethral resection. Specimens of normal human ureters were obtained surgically. Singly dispersed TCC cells grew in 0.3% agarose semisolid medium with a cloning efficiency ranging from 0.02% to 0.71%. Singly dispersed normal ureteral urothelial cells under the same conditions did not form colonies in 0.3% agarose. Neither singly dispersed TCC nor normal urothelial cells formed colonies when plated on collagen-gel substrates. In primary explant culture, normal human urothelial cells grew rapidly, to form tightly adherent flat sheets of apparently nonmotile cells. Autoradiographic labeling with 3H-thymidine of growing cultures of normal urothelial cells showed cell division primarily in the zones of growth near the explant. Outgrowth of TCC from primary explants was loosely adherent. One TCC explant culture gave rise to a continuous suspension culture. Numerous multilayered cellular formations of fronds, nodules, and "walls" were seen around the periphery of TCC explant colonies. Autoradiography showed that these multilayered areas of TCC growth contained actively dividing cells. The altered ability of papillary TCC to form superficial multilayered formations in vitro distinguishes them from normal human urothelium and reflects the morphologic characteristic of this tumor type in vivo.
Full text
PDF


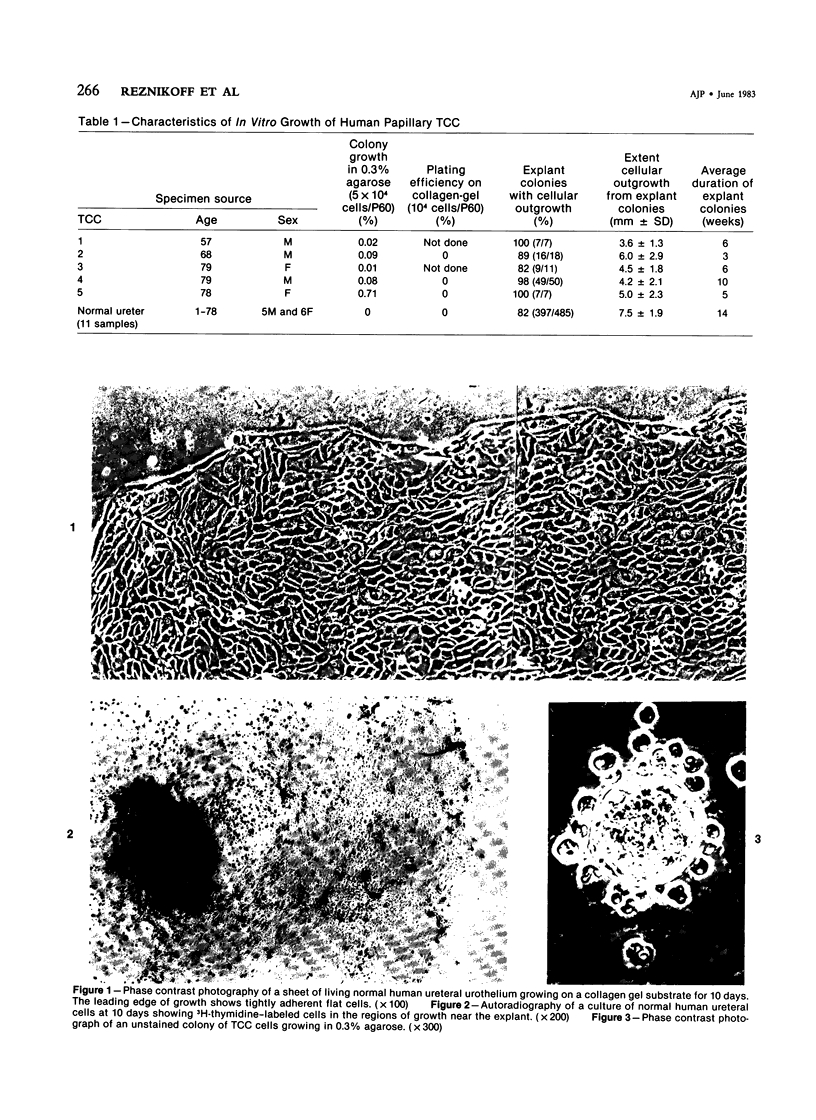

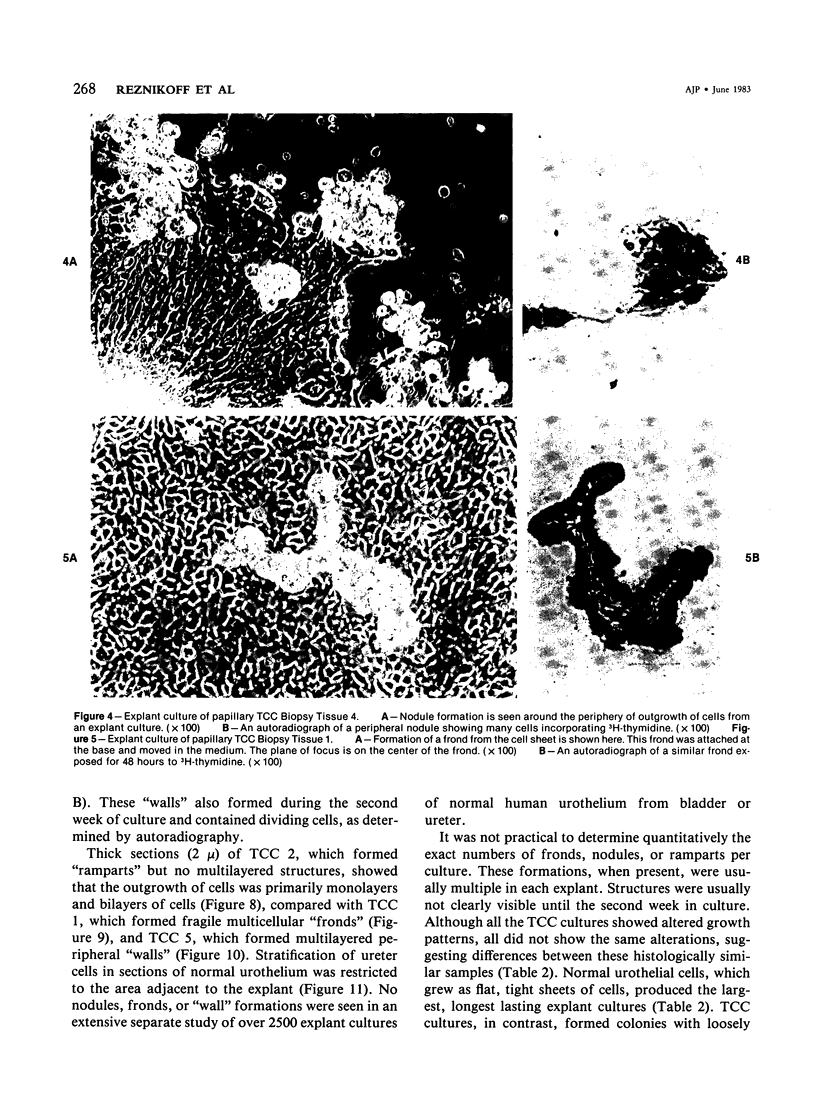
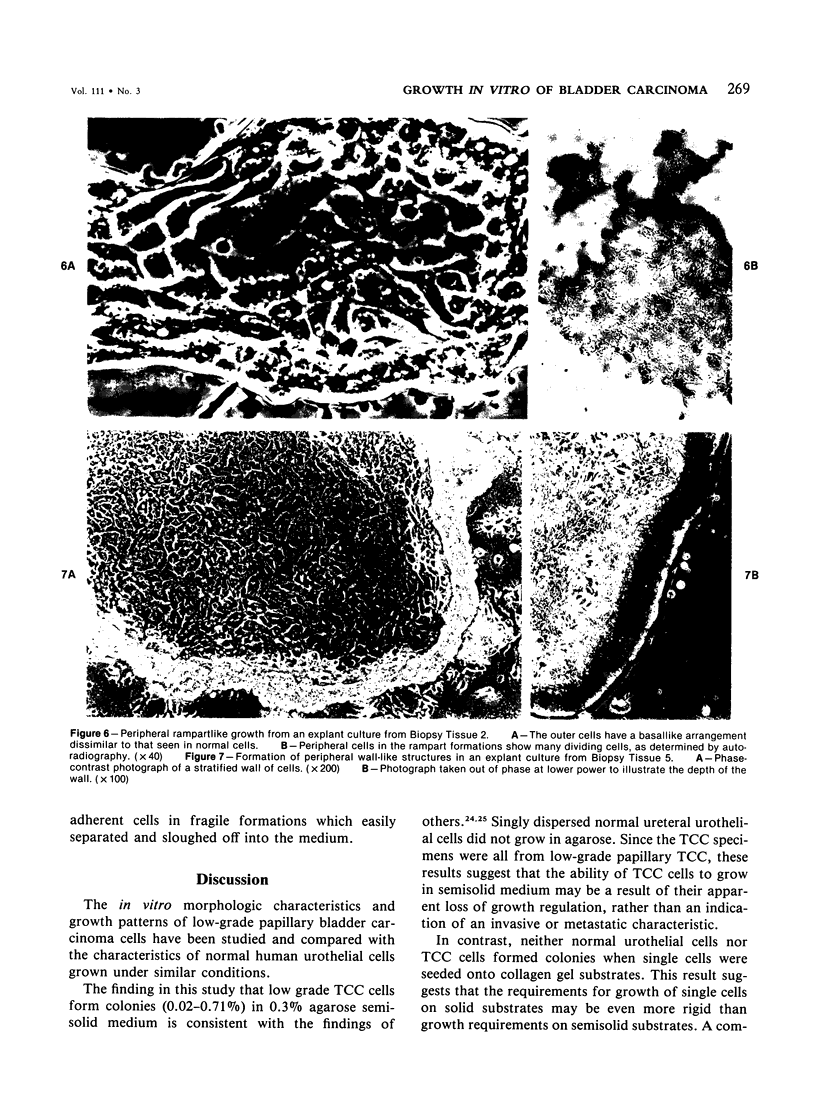



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abaza N. A., Leighton J., Zajac B. A. Clinical bladder cancer in sponge matrix tissue culture: procedures for collection, cultivation, and assessment of viability. Cancer. 1978 Sep;42(3):1364–1374. doi: 10.1002/1097-0142(197809)42:3<1364::aid-cncr2820420347>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Buick R. N., Stanisic T. H., Fry S. E., Salmon S. E., Trent J. M., Krasovich P. Development of an agar-methyl cellulose clonogenic assay for cells in transitional cell carcinoma of the human bladder. Cancer Res. 1979 Dec;39(12):5051–5056. [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlapowski F. J., Haynes L. The growth and differentiation of transitional epithelium in vitro. J Cell Biol. 1979 Dec;83(3):605–614. doi: 10.1083/jcb.83.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRMANN R. L., GEY G. O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956 Jun;16(6):1375–1403. [PubMed] [Google Scholar]
- Elliott A. Y., Bronson D. L., Cervenka J., Stein N., Fraley E. E. Properties of cell lines established from transitional cell cancers of the human urinary tract. Cancer Res. 1977 May;37(5):1279–1289. [PubMed] [Google Scholar]
- Elliott A. Y., Bronson D. L., Stein N., Fraley E. E. In vitro cultivation of epithelial cells derived from tumors of the human urinary tract. Cancer Res. 1976 Feb;36(2 Pt 1):365–369. [PubMed] [Google Scholar]
- Fogh J. Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors. Natl Cancer Inst Monogr. 1978 Dec;(49):5–9. [PubMed] [Google Scholar]
- Friedman E. A., Higgins P. J., Lipkin M., Shinya H., Gelb A. M. Tissue culture of human epithelial cells from benign colonic tumors. In Vitro. 1981 Jul;17(7):632–644. doi: 10.1007/BF02618462. [DOI] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Hastings R. J., Franks L. M. Chromosome pattern, growth in agar and tumorigenicity in nude mice of four human bladder carcinoma cell lines. Int J Cancer. 1981 Jan 15;27(1):15–21. doi: 10.1002/ijc.2910270104. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Larner J., Sato G. Hormonal growth control of cells in culture. In Vitro. 1978 Jan;14(1):23–30. doi: 10.1007/BF02618171. [DOI] [PubMed] [Google Scholar]
- Hicks R. M., Chowaniec J. Experimental induction, histology, and ultrastructure of hyperplasia and neoplasia of the urinary bladder epithelium. Int Rev Exp Pathol. 1978;18:199–280. [PubMed] [Google Scholar]
- Hicks R. M. Multistage carcinogenesis in the urinary bladder. Br Med Bull. 1980 Jan;36(1):39–46. doi: 10.1093/oxfordjournals.bmb.a071611. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. A., Hicks R. M., Berry R. J., Milroy E. Organ culture of normal human bladder: choice of starting material and culture characteristics. Methods Cell Biol. 1980;21B:257–285. doi: 10.1016/s0091-679x(08)60687-1. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Bubeník Human urinary bladder carcinoma cell line (T24) in long-term culture: chromosomal studies on a wild population and derived sublines. Neoplasma. 1977;24(3):319–326. [PubMed] [Google Scholar]
- Marshall C. J., Franks L. M., Carbonell A. W. Markers of neoplastic transformation in epithelial cell lines derived from human carcinomas. J Natl Cancer Inst. 1977 Jun;58(6):1743–1751. doi: 10.1093/jnci/58.6.1743. [DOI] [PubMed] [Google Scholar]
- Murphy W. M., Soloway M. S., Lin C. J. Morphologic effects of thio-TEPA on mouse urothelium. Acta Cytol. 1977 Sep-Oct;21(5):701–704. [PubMed] [Google Scholar]
- Pauli B. U., Anderson S. N., Memoli V. A., Kuettner K. E. The isolation and characterization in vitro of normal epithelial cells, endothelial cells and fibroblasts from rat urinary bladder. Tissue Cell. 1980;12(3):419–436. doi: 10.1016/0040-8166(80)90033-6. [DOI] [PubMed] [Google Scholar]
- Stoner G. D., Katoh Y., Foidart J. M., Trump B. F., Steinert P. M., Harris C. C. Cultured human bronchial epithelial cells: blood group antigens, keratin, collagens, and fibronectin. In Vitro. 1981 Jul;17(7):577–587. doi: 10.1007/BF02618455. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Lui G. M., Gospodarowicz D. Morphological appearance, growth behavior and migratory activity of human tumor cells maintained on extracellular matrix versus plastic. Cell. 1980 Mar;19(3):607–616. doi: 10.1016/s0092-8674(80)80037-7. [DOI] [PubMed] [Google Scholar]
- Williams R. D. Human urologic cancer cell lines. Invest Urol. 1980 Mar;17(5):359–363. [PubMed] [Google Scholar]
- Yang J., Elias J. J., Petrakis N. L., Wellings S. R., Nandi S. Effects of hormones and growth factors on human mammary epithelial cells in collagen gel culture. Cancer Res. 1981 Mar;41(3):1021–1027. [PubMed] [Google Scholar]
- Yang J., Guzman R., Richards J., Jentoft V., DeVault M. R., Wellings S. R., Nandi S. Primary culture of human mammary epithelial cells embedded in collagen gels. J Natl Cancer Inst. 1980 Aug;65(2):337–343. [PubMed] [Google Scholar]
- Yang N. S., Kube D., Park C., Furmanski P. Growth of human mammary epithelial cells on collagen gel surfaces. Cancer Res. 1981 Oct;41(10):4093–4100. [PubMed] [Google Scholar]
- van der Bosch J., Masui H., Sato G. Growth characteristics of primary tissue cultures from heterotransplanted human colorectal carcinomas in serum-free medium. Cancer Res. 1981 Feb;41(2):611–618. [PubMed] [Google Scholar]