Abstract
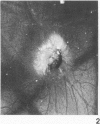
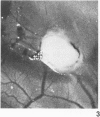
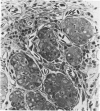
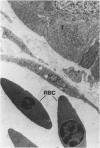
Neoplastic tumors are able to elicit the ingrowth of new capillaries, a process known as angiogenesis. The chorioallantoic membrane (CAM) of chicken embryos was used in an assay for this response, and normal mammary glands and various mammary growths from GR mice, including plaques, hyperplasic alveolar nodules, and hormone-dependent and hormone-independent tumors were tested. Fifteen percent of the male mammary glands tested were positive, as were 28% of the resting female mammary glands. Fifty percent of the plaques and 63% of the hyperplastic alveolar nodules tested induced neovascularization. Eighty percent of the hormone-dependent tumors and 97% of the hormone-independent tumors tested elicited angiogenesis. A fine-structural study revealed that capillaries invaded to within less than 0.5 microns of the tumor cells, but no penetration of tumor cells through the basal lamina was observed. Positive responses were directly correlated with the neoplastic potential of the tissues tested, indicating that angiogenesis can predict mammary gland growths most likely to become malignant.
Full text
PDF

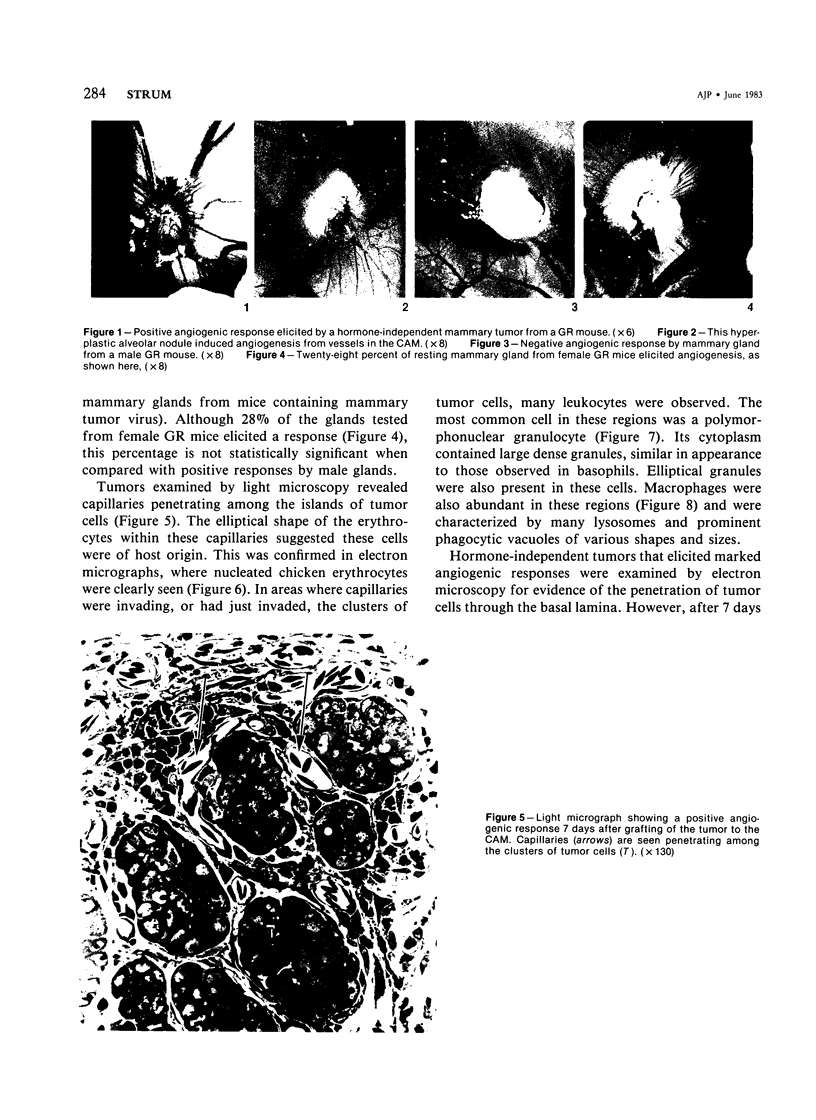

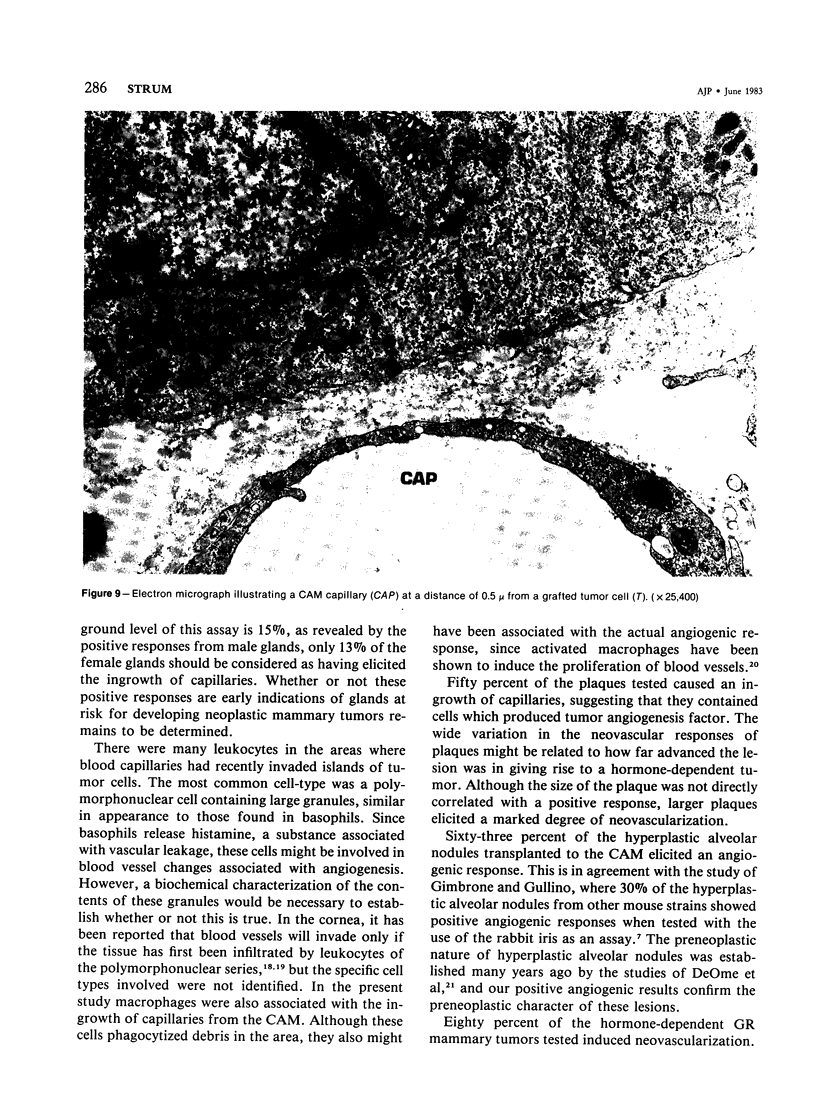

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aidells B. D., Daniel C. W. Hormone-dependent mammary tumors in strain GR/A mice. IV. Origin and progression. J Natl Cancer Inst. 1978 Jun;60(6):1345–1350. doi: 10.1093/jnci/60.6.1345. [DOI] [PubMed] [Google Scholar]
- Brem S. S., Gullino P. M., Medina D. Angiogenesis: a marker for neoplastic transformation of mammary papillary hyperplasia. Science. 1977 Mar 4;195(4281):880–882. doi: 10.1126/science.402692. [DOI] [PubMed] [Google Scholar]
- Brem S. S., Jensen H. M., Gullino P. M. Angiogenesis as a marker of preneoplastic lesions of the human breast. Cancer. 1978 Jan;41(1):239–244. doi: 10.1002/1097-0142(197801)41:1<239::aid-cncr2820410133>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- FOULDS L. The experimental study of tumor progression: a review. Cancer Res. 1954 Jun;14(5):327–339. [PubMed] [Google Scholar]
- Folkman J., Merler E., Abernathy C., Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971 Feb 1;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Proceedings: Tumor angiogenesis factor. Cancer Res. 1974 Aug;34(8):2109–2113. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1974;19(0):331–358. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. III. Studies related to the vasoproliferative capability of polymorphonuclear leukocytes and lymphocytes. Am J Pathol. 1976 Jan;82(1):157–170. [PMC free article] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. Am J Pathol. 1975 Jun;79(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Gullino P. M. Neovascularization induced by intraocular xenografts of normal, preneoplastic, and neoplastic mouse mammary tissues. J Natl Cancer Inst. 1976 Feb;56(2):305–318. doi: 10.1093/jnci/56.2.305. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Leapman S. B., Cotran R. S., Folkman J. Tumor angiogenesis: iris neovascularization at a distance from experimental intraocular tumors. J Natl Cancer Inst. 1973 Jan;50(1):219–228. doi: 10.1093/jnci/50.1.219. [DOI] [PubMed] [Google Scholar]
- Greenblatt M., Shubi P. Tumor angiogenesis: transfilter diffusion studies in the hamster by the transparent chamber technique. J Natl Cancer Inst. 1968 Jul;41(1):111–124. [PubMed] [Google Scholar]
- Mühlbock O. Note on a new inbred mouse-strain GR-A. Eur J Cancer. 1965 Oct;1(2):123–124. doi: 10.1016/0014-2964(65)90003-4. [DOI] [PubMed] [Google Scholar]
- Pitelka D. R., Hamamoto S. T., Taggart B. N. Basal lamina and tissue recognition in malignant mammary tumors. Cancer Res. 1980 May;40(5):1600–1611. [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nie R. Behaviour and morphology of pregnancy responsive mammary tumours in mice. Pathol Eur. 1967;2(4):357–373. [PubMed] [Google Scholar]
- van Nie R., Thung P. J. Responsiveness of mouse mammary tumours to pregnancy. Eur J Cancer. 1965 Jun;1(1):41–50. doi: 10.1016/0014-2964(65)90078-2. [DOI] [PubMed] [Google Scholar]