Abstract
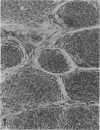
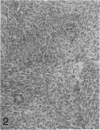
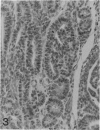
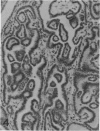
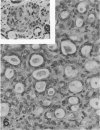
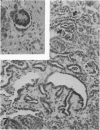
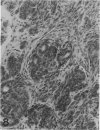
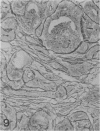
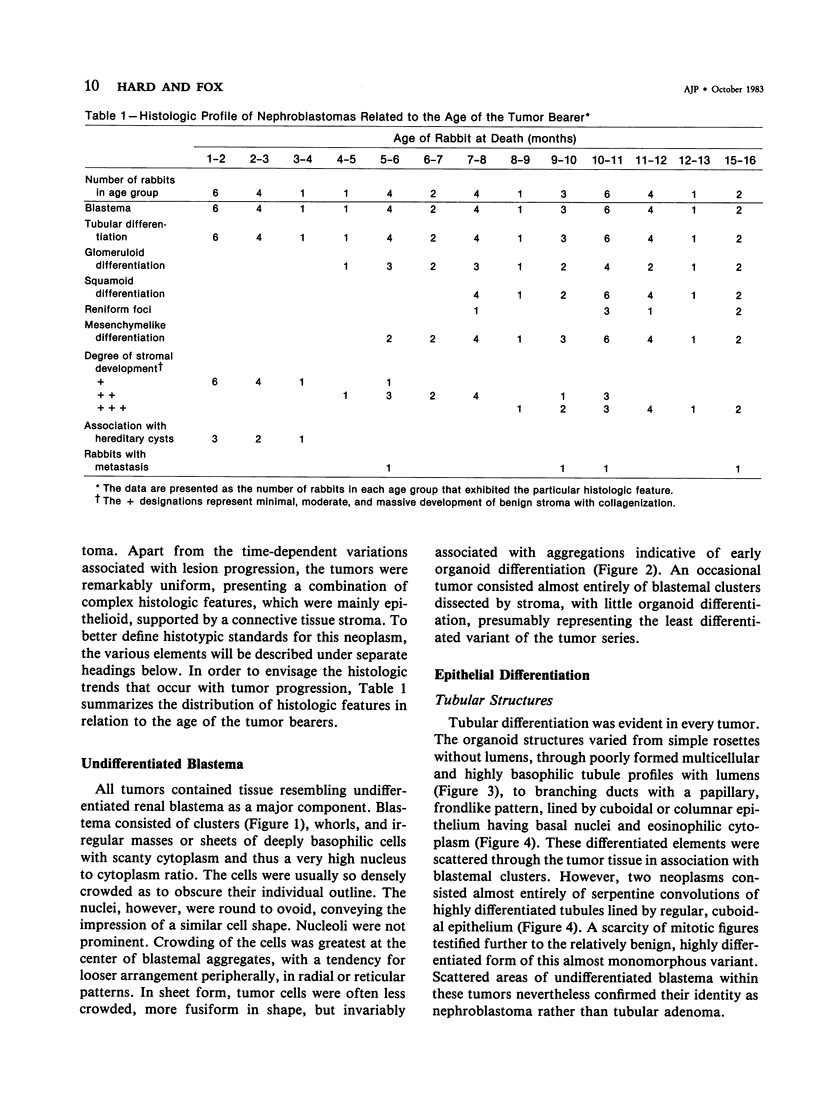
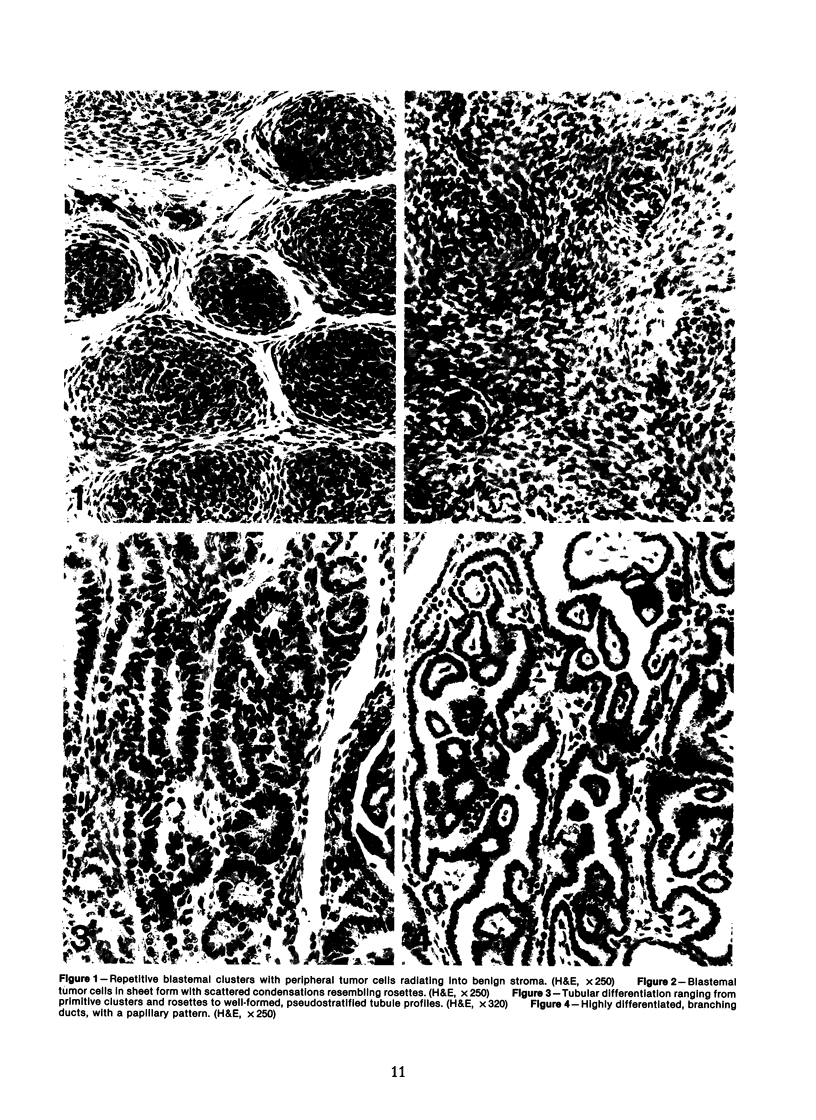
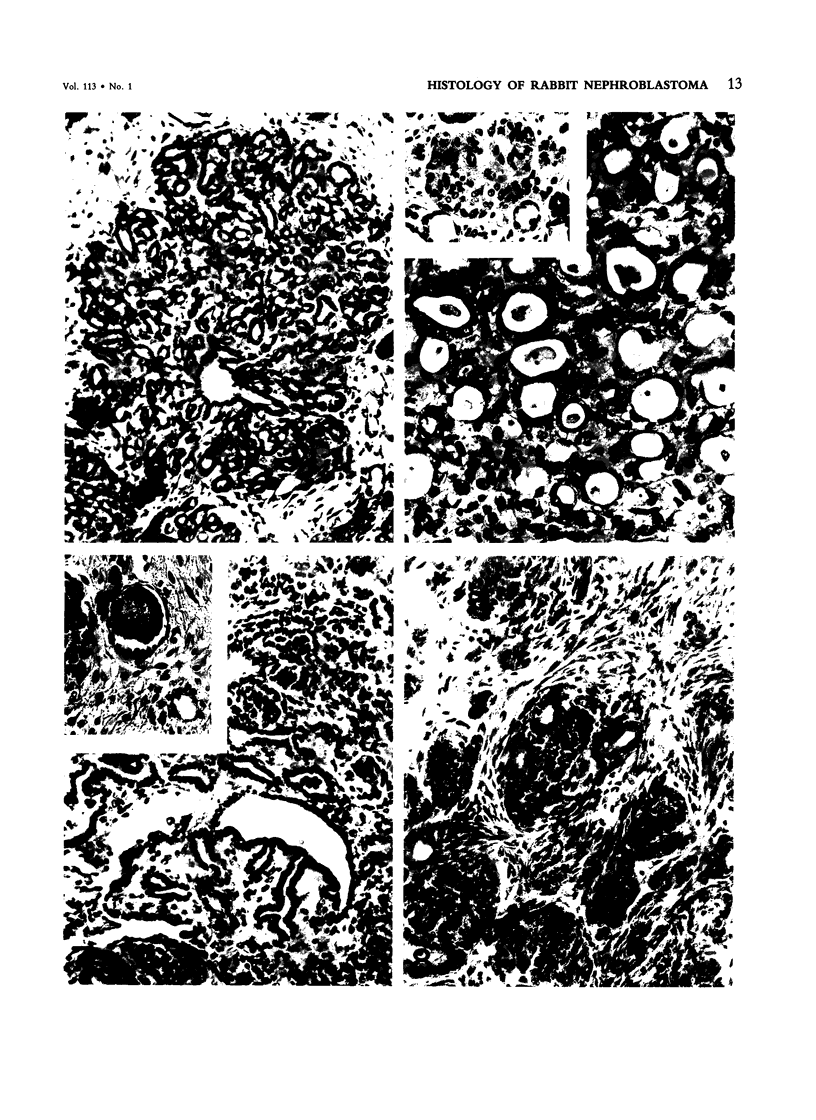
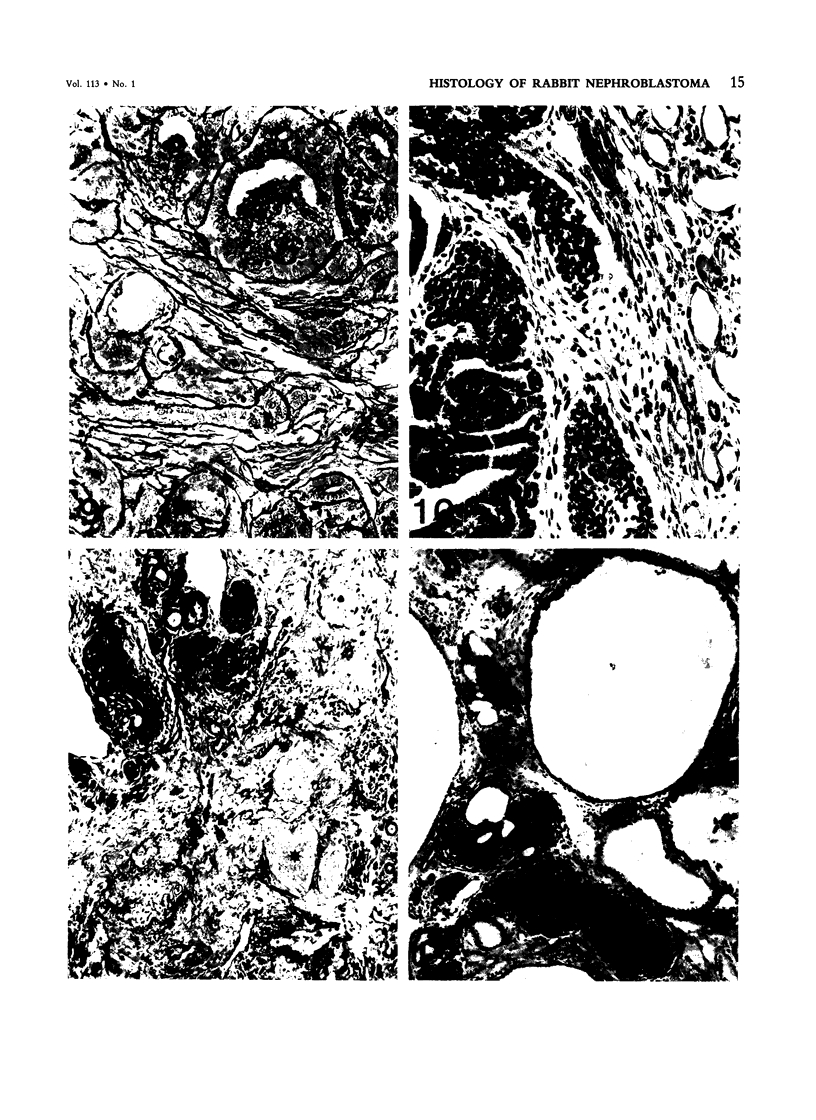
The histologic features of 63 renal tumors induced in 39 rabbits of two partially inbred strains, IIIVO/J and WH/J, by transplacental exposure to N-ethylnitrosourea (ENU) were analyzed. All tumors in the series conformed to nephroblastoma, permitting the establishment of histologic standards for this neoplasm in the rabbit as well as observations on tumor progression. Essentially, nephroblastoma proved to be predominantly an epithelial tumor identifying with metanephrogenic blastema, which was presumed to be the tissue of origin during fetal development. The outstanding features comprised clusters or sheets of undifferentiated blastemalike tissue and differentiation along the epithelial pathway into tubular profiles and structures suggestive of primitive, nonvascularized glomeruli. The latter were frequently of a complex nature, with a papillary configuration. On the other hand, no definitive evidence of bipotential differentiation into malignant secondary mesenchyme was found, there being no recognizable areas of fibrosarcomatous elements or specialized connective tissue such as smooth or striated muscle, adipose tissue, cartilage, or osteoid. Mesenchymelike fascicular disposition of neoplastic cells between blastemal clusters was an acquired feature seen in advanced tumors but not in small early lesions. By light microscopy alone it was not possible to determine whether this represented a conformational change of tumor cells or true bipotential differentiation into neoplastic secondary mesenchyme. However, the reticulin pattern was not characteristic of sarcoma. A conspicuous feature accompanying the growth and development of tumors was the magnitude of host fibrous reaction discernible only as a simple ramifying stroma in the earliest lesions but attaining impressive proportions both within and around the tumor with advancing age. Increasing collagen formation appeared to be associated with ischemic necrosis of tumor tissue. Other features of advanced tumors were the presence of discrete foci of differentiated tubular structures suggestive of mature medullary elements and small islands of squamoid differentiation. Metastases occurred only in rabbits of strain IIIVO/J, which had been subjected to a single dose of the carcinogen, representing an incidence in this subgroup of 25%. Nephroblastomas resulting from transplacental induction in IIIVO/J rabbits, particularly by single, high doses of ENU, appear to provide a suitable model for the predominant histologic form of the Wilms' tumor complex in man.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckwith J. B., Palmer N. F. Histopathology and prognosis of Wilms tumors: results from the First National Wilms' Tumor Study. Cancer. 1978 May;41(5):1937–1948. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Bolande R. P., Brough A. J., Izant R. J., Jr Congenital mesoblastic nephroma of infancy. A report of eight cases and the relationship to Wilms' tumor. Pediatrics. 1967 Aug;40(2):272–278. [PubMed] [Google Scholar]
- Bove K. E., McAdams A. J. The nephroblastomatosis complex and its relationship to Wilms' tumor: a clinicopathologic treatise. Perspect Pediatr Pathol. 1976;3:185–223. [PubMed] [Google Scholar]
- Chatten J. Epithelial differentiation in Wilms' tumor: a clinicopathologic appraisal. Perspect Pediatr Pathol. 1976;3:225–254. [PubMed] [Google Scholar]
- FEKETE E. A morphological study of the ovaries of virgin mice of eight inbred strains showing quantitative differences in their hormone producing components. Anat Rec. 1953 Sep;117(1):93–113. doi: 10.1002/ar.1091170108. [DOI] [PubMed] [Google Scholar]
- Fox R. R., Diwan B. A., Meier H. Transplacental induction of primary renal tumors in rabbits treated with 1-ethyl-1-nitrosourea. J Natl Cancer Inst. 1975 Jun;54(6):1439–1448. doi: 10.1093/jnci/54.6.1439. [DOI] [PubMed] [Google Scholar]
- Fox R. R., Krinsky W. L., Crary D. D. Hereditary cortical renal cysts in the rabbit. J Hered. 1971 Mar-Apr;62(2):105–109. doi: 10.1093/oxfordjournals.jhered.a108132. [DOI] [PubMed] [Google Scholar]
- Fox R. R., Meier H., Bedigian H. G., Crary D. D. Genetics of transplacentally induced teratogenic and carcinogenic effects in rabbits treated with N-nitroso-N-ethylurea. J Natl Cancer Inst. 1982 Dec;69(6):1411–1417. [PubMed] [Google Scholar]
- Fox R. R., Meier H., Crary D. D. Genetic predisposition to tumors in the rabbit. Naturwissenschaften. 1971 Sep;58(9):457–457. doi: 10.1007/BF00624625. [DOI] [PubMed] [Google Scholar]
- Fox R. R., Meier H., Pottathil R., Bedigian H. G. Transplacental teratogenic and carcinogenic effects in rabbits chronically treated with N-ethyl-N-nitrosourea. J Natl Cancer Inst. 1980 Sep;65(3):607–614. [PubMed] [Google Scholar]
- Haas J. E., Palmer N. F., Weinberg A. G., Beckwith J. B. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Hum Pathol. 1981 Jul;12(7):646–657. doi: 10.1016/s0046-8177(81)80050-0. [DOI] [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Cellular analysis of renal neoplasia: induction of renal tumors in dietary-conditioned rats by dimethylnitrosamine, with a reappraisal of morphological characteristics. Cancer Res. 1970 Nov;30(11):2796–2805. [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Cellular analysis of renal neoplasia: light microscope study of the development of interstitial lesions induced in the rat kidney by a single carcinogenic dose of dimethylnitrosamine. Cancer Res. 1970 Nov;30(11):2806–2815. [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Ultrastructural analysis of renal mesenchymal tumor induced in the rat by dimethylnitrosamine. Cancer Res. 1971 Mar;31(3):348–365. [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Ultrastructural study of the development of interstitial lesions leading to mesenchymal neoplasia induced in the rat renal cortex by dimethylnitrosamine. Cancer Res. 1971 Mar;31(3):337–347. [PubMed] [Google Scholar]
- Hard G. C., Grasso P. Nephroblastoma in the rat: histology of a spontaneous tumor, identity with respect to renal mesenchymal neoplasms, and a review of previously recorded cases. J Natl Cancer Inst. 1976 Aug;57(2):323–329. doi: 10.1093/jnci/57.2.323. [DOI] [PubMed] [Google Scholar]
- Hard G. C., Noble R. L. Occurrence, transplantation, and histologic characteristics of nephroblastoma in the Nb hooded rat. Invest Urol. 1981 Mar;18(5):371–376. [PubMed] [Google Scholar]
- Hard G. C., Noble R. L. Spontaneous rat nephroblastoma: ultrastructure of a transplant line. Arch Pathol Lab Med. 1982 Aug;106(8):418–422. [PubMed] [Google Scholar]
- Jasmin G., Riopelle J. L. Nephroblastomas induced in ovariectomized rats by dimethylbenzanthracene. Cancer Res. 1970 Feb;30(2):321–326. [PubMed] [Google Scholar]
- Jurgelski W., Jr, Hudson P., Falk H. L. Tissue differentiation and susceptibility to embryonal tumor induction by ethylnitrosourea in the opossum. Natl Cancer Inst Monogr. 1979 May;(51):123–158. [PubMed] [Google Scholar]
- Mirvish S. S. Kinetics of nitrosamide formation from alkylureas, N-alkylurethans, and alkylguanidines: possible implications for the etiology of human gastric cancer. J Natl Cancer Inst. 1971 Jun;46(6):1183–1193. [PubMed] [Google Scholar]
- Stavrou D., Hänichen T. Oncogene Wirkung von Athylnitrosoharnstoff beim kaninchen während der pränatalen Periode. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1975 Oct 27;84(2):207–215. doi: 10.1007/BF00304046. [DOI] [PubMed] [Google Scholar]
- Turusov V. S., Alexandrov V. A., Timoshenko I. V. Nephroblastoma and renal mesenchymal tumor induced in rats by N-nitrosoethyl- and N-nitrosomethylurea. Neoplasma. 1980;27(3):229–235. [PubMed] [Google Scholar]
- Watts S. L., Smith R. E. Pathology of chickens infected with avian nephoblastoma virus MAV-2(N). Infect Immun. 1980 Feb;27(2):501–512. doi: 10.1128/iai.27.2.501-512.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]