Abstract
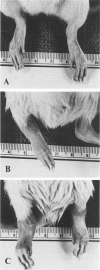
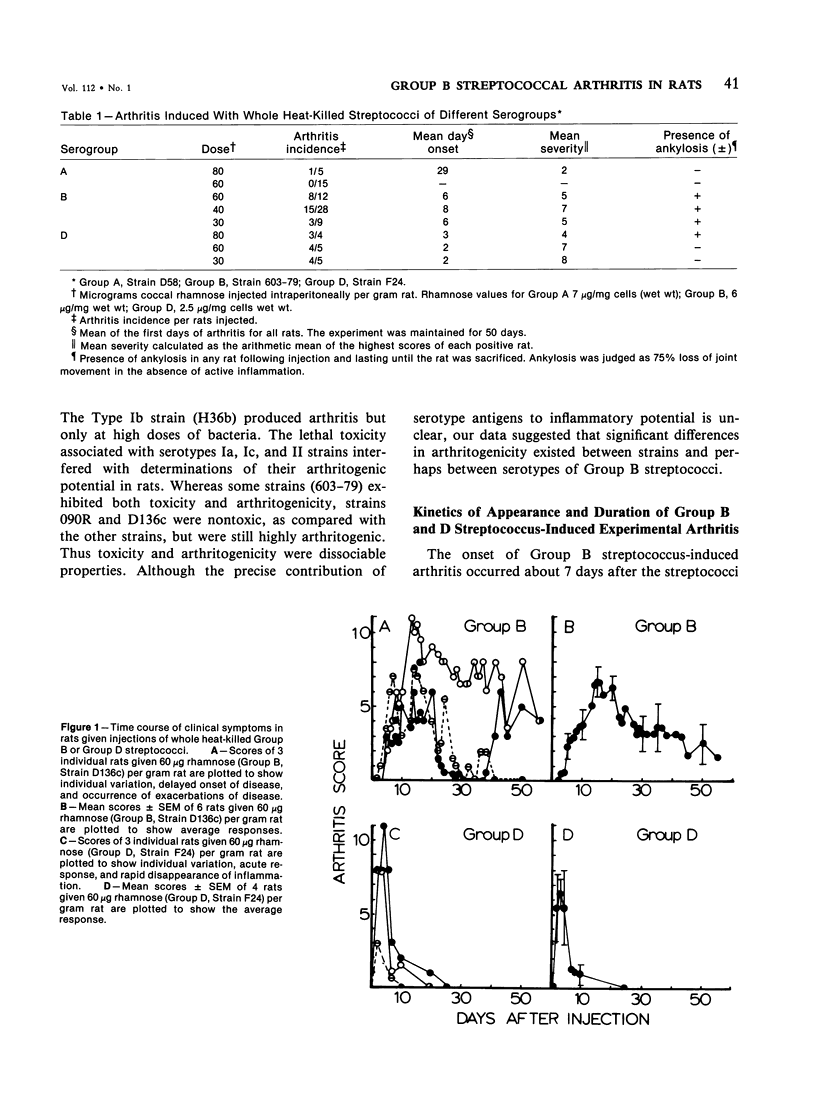
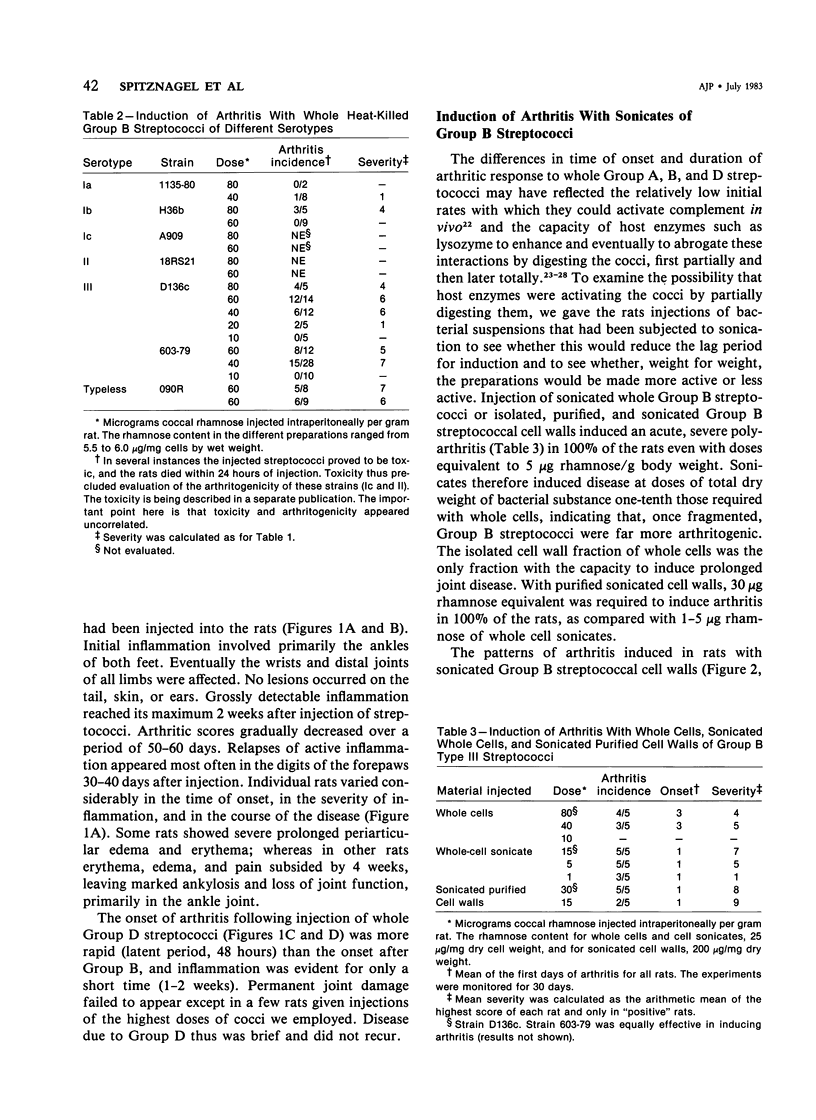
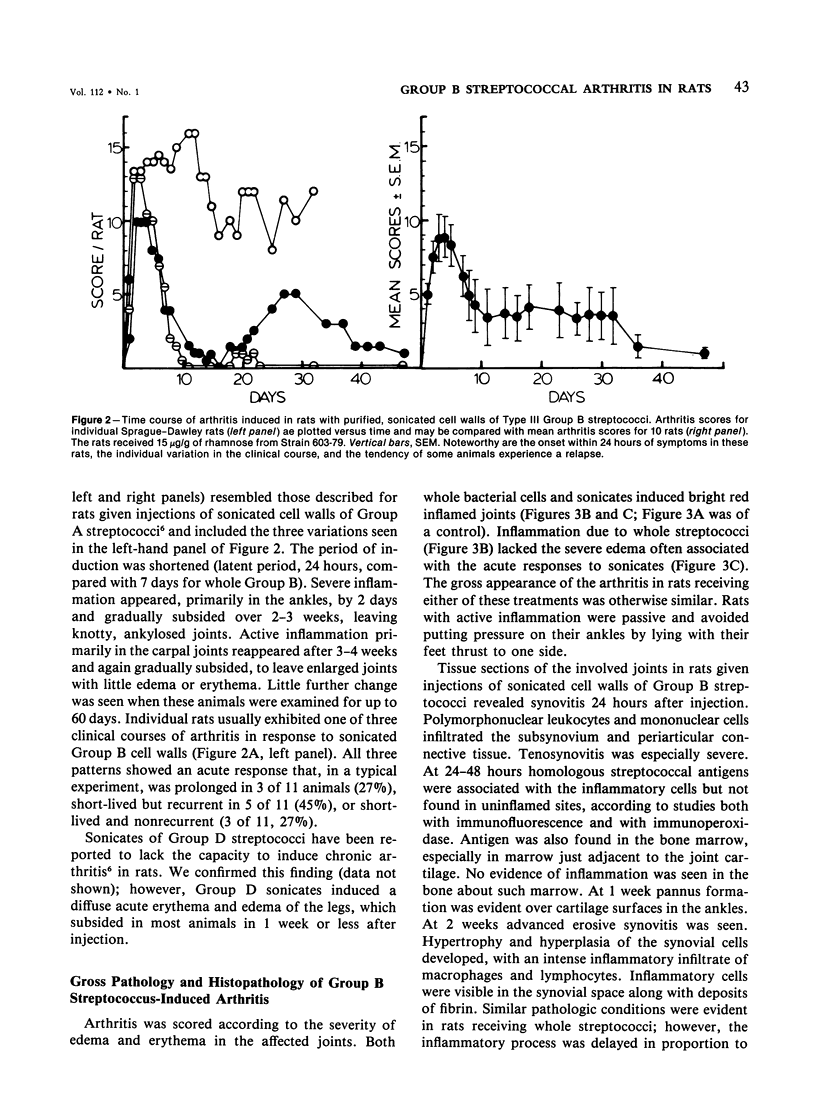
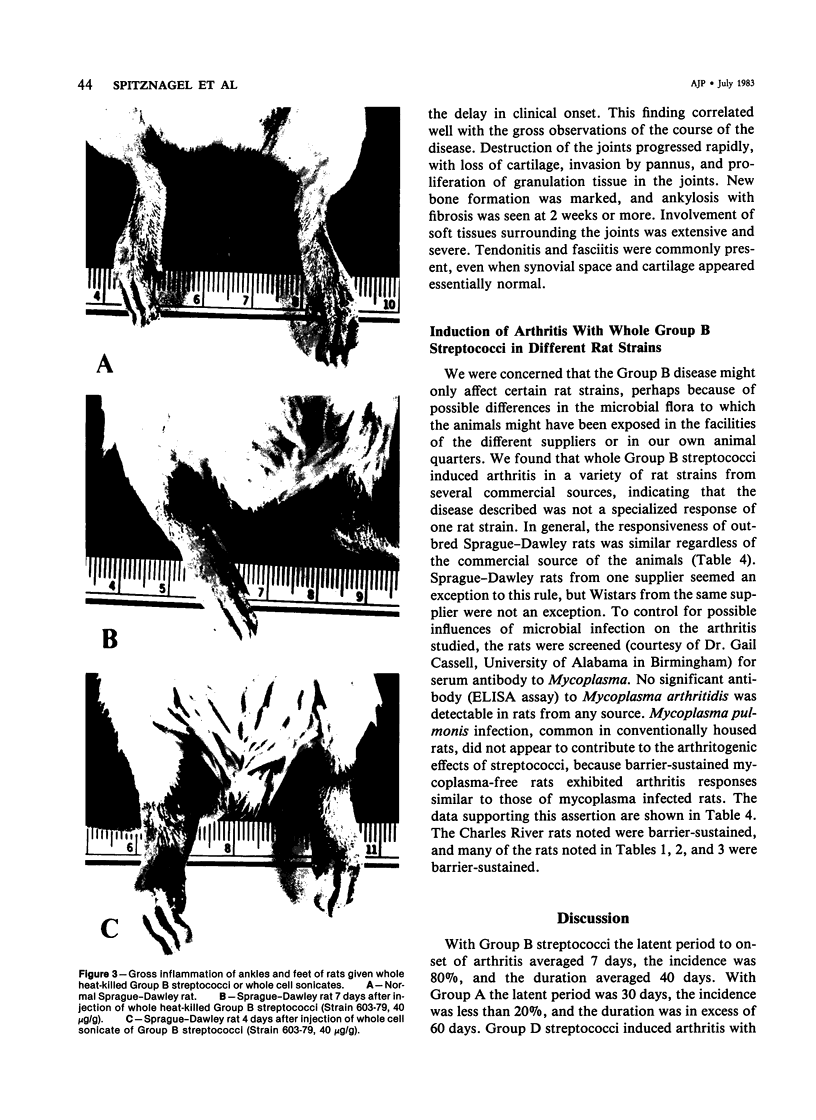
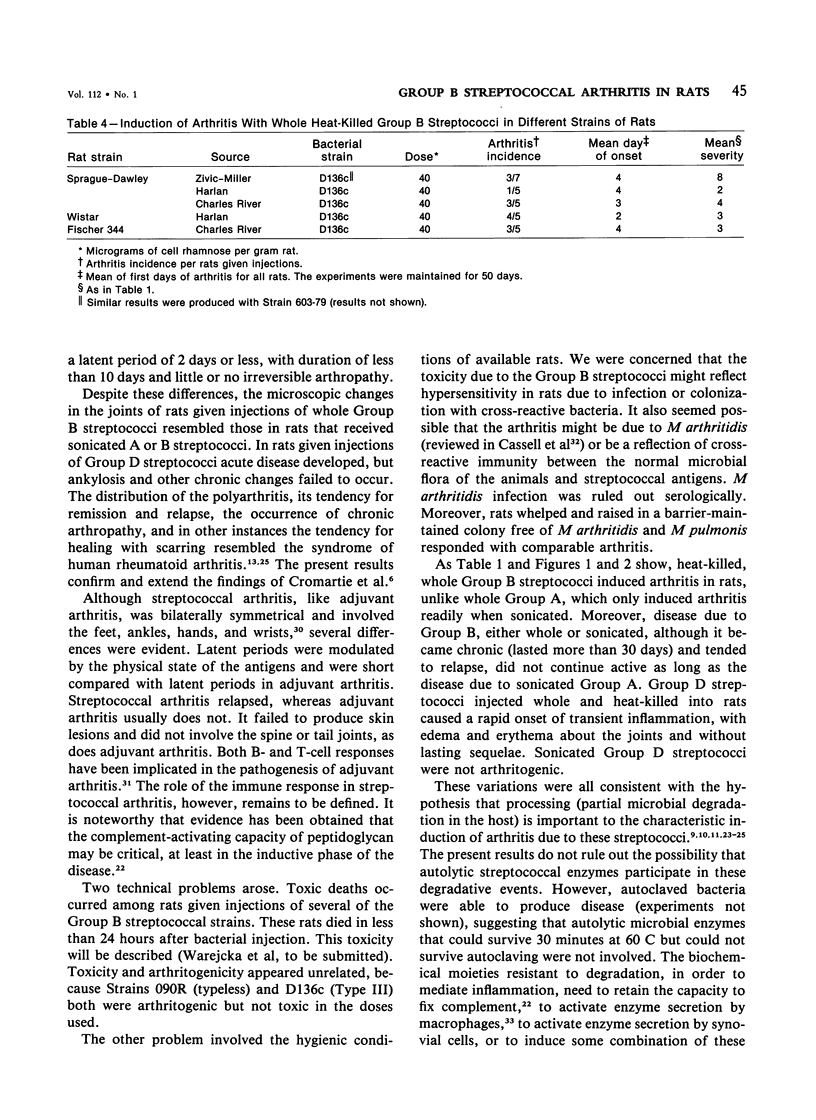
Heat-killed streptococci of Groups A, B, and D injected intraperitoneally into Sprague-Dawley rats induced arthritis. The histopathologic features of the arthritis were those of erosive synovitis. Early acute lesions were associated with deposits of streptococcal antigens. The serogroups and the physical state of the streptococci determined the incidence, the time of onset, the duration, and the severity of the disease, the severity being a blend of degree of inflammation, tendency to relapse, and occurrence of ankylosis. Whole Group A usually failed to induce arthritis. Group A disrupted with sonication regularly induced arthritis after a 24-hour latent period. The disease lasted over 60 days and caused ankylosis. Whole Group B regularly induced arthritis but only after a latent period of 6-8 days. The disease lasted over 40 days and caused ankylosed joints. With sonicated Group B a similar disease was induced, except that, as with sonicated Group A, the latent period was 24 hours. Whole Group D induced disease after a latent period of 48 hours. The arthritis lasted only 2 weeks and was transient. In contrast to its effects on Group A and B cocci, sonication of Group D abrogated its capacity to induce arthritis. It is postulated that for whole streptococci, in contrast to sonicated streptococci, arthritogenicity depends on the sensitivity of the cocci to initial processing in vivo. Processing may be partial digestion by enzymes of phagocytes. Cocci such as those of Group A that are insensitive to processing, injected whole, tend not to cause arthritis, but when they do cause disease, it is chronic. A coccus, such as one of Group D, that is very sensitive to processing produces a transient arthritis after a short latent period, while a coccus of intermediate sensitivity, such as one of Group B, induces disease only after a substantial latent period, and the disease is severe and chronic. The nature of processing remains to be determined.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderle S. K., Greenblatt J. J., Cromartie W. J., Clark R., Schwab J. H. Modulation of the susceptibility of inbred and outbred rats to arthritis induced by cell walls of group A streptococci. Infect Immun. 1979 Aug;25(2):484–490. doi: 10.1128/iai.25.2.484-490.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub E. M., Wannamaker L. W. The fate of group A streptococci following phagocytosis. In vitro phagocytic studies of isotope-labeled streptococci. J Immunol. 1967 Dec;99(6):1099–1105. [PubMed] [Google Scholar]
- Chang Y. H., Pearson C. M., Chedid L. Adjuvant polyarthritis. V. Induction by N-acetylmuramyl-L-alanyl-D-isoglutamine, the smallest peptide subunit of bacterial peptidoglycan. J Exp Med. 1981 Apr 1;153(4):1021–1026. doi: 10.1084/jem.153.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Graham R., Russell G., Krane S. M. Collagenase production by rheumatoid synovial cells: stimulation by a human lymphocyte factor. Science. 1977 Jan 14;195(4274):181–183. doi: 10.1126/science.188134. [DOI] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis H. A., Miller S. E., Wheat R. W. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infect Immun. 1976 May;13(5):1459–1466. doi: 10.1128/iai.13.5.1459-1466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Ginsburg I. Mechanisms of cell and tissue injury induced by group A streptococci: relation to poststreptococcal sequelae. J Infect Dis. 1972 Oct;126(4):419–456. doi: 10.1093/infdis/126.4.419. [DOI] [PubMed] [Google Scholar]
- Glick A. D., Ranhand J. M., Cole R. M. Degradation of group A streptococcal cell walls by egg-white lysozyme and human lysosomal enzymes. Infect Immun. 1972 Sep;6(3):403–413. doi: 10.1128/iai.6.3.403-413.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadler N. M. A pathogenetic model for erosive synovitis: lessons from animal arthritides. Arthritis Rheum. 1976 Mar-Apr;19(2):256–266. doi: 10.1002/art.1780190222. [DOI] [PubMed] [Google Scholar]
- Hadler N. M., Granovetter D. A. Phlogistic properties of bacterial debris. Semin Arthritis Rheum. 1978 Aug;8(1):1–16. doi: 10.1016/0049-0172(78)90031-8. [DOI] [PubMed] [Google Scholar]
- Hamerman D. New thoughts on the pathogenesis of rheumatoid arthritis. Am J Med. 1966 Jan;40(1):1–9. doi: 10.1016/0002-9343(66)90181-1. [DOI] [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H. Persistence of group a streptococcal cell walls related to chronic inflammation of rabbit dermal connective tissue. J Exp Med. 1967 Jun 1;125(6):1137–1148. doi: 10.1084/jem.125.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSON C. M., WOOD F. D. Studies of arthritis and other lesions induced in rats by the injection of mycobacterial adjuvant. VII. Pathologic details of the arthritis and spondylitis. Am J Pathol. 1963 Jan;42:73–95. [PMC free article] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. Pathogenesis of the chronic inflammatory lesion induced by group A streptococcal cell walls. Lab Invest. 1974 May;30(5):568–581. [PubMed] [Google Scholar]
- Patterson M. J., El Batool Hafeez A. Group B streptococci in human disease. Bacteriol Rev. 1976 Sep;40(3):774–792. doi: 10.1128/br.40.3.774-792.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryzwansky K. B., Martin L. E., Spitznagel J. K. Immunocytochemical localization of myeloperoxidase, lactoferrin, lysozyme and neutral proteases in human monocytes and neutrophilic granulocytes. J Reticuloendothel Soc. 1978 Sep;24(3):295–310. [PubMed] [Google Scholar]
- Rosenthale M. E., Capetola R. J. Adjuvant arthritis: immunopathological and hyperalgesic features. Fed Proc. 1982 Jul;41(9):2577–2582. [PubMed] [Google Scholar]
- Schwab J. H., Allen J. B., Anderle S. K., Dalldorf F., Eisenberg R., Cromartie W. J. Relationship of complement to experimental arthritis induced in rats with streptococcal cell walls. Immunology. 1982 May;46(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Cromartie W. J., Ohanian S. H., Craddock J. G. Association of experimental chronic arthritis with the persistence of group A streptococcal cell walls in the articular tissue. J Bacteriol. 1967 Nov;94(5):1728–1735. doi: 10.1128/jb.94.5.1728-1735.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood F. D., Pearson C. M., Tanaka A. Capacity of mycobacterial wax D and its subfractions to induce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol. 1969;35(5):456–467. doi: 10.1159/000230198. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]